Activity 11.5
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Conductivity of Liquids
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to explore which liquids can conduct electricity and why. Can anyone tell me what they remember about conductors and insulators?

Conductors allow electricity to flow through, while insulators do not.

Exactly! Now, can we classify some liquids? How about lemon juice or vinegar?

I remember from the experiment that they glowed the bulb, so they must be good conductors.

Great observation! And did you know there's a memory aid for remembering good conductors? It’s 'Lively Acidic Salts'! What does it remind you of?

Acids and salts conduct electricity better!

Exactly! The ability to conduct electricity shows us there's a flow of ions in the solution.

Now let’s summarize: Solutions that contain ions, like acidic or salty solutions, conduct electricity well!
Chemical Reactions in Conducting Solutions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand conductivity, let’s delve into what happens chemically when current flows through a liquid. Can anyone explain the changes we might expect?

I think gas bubbles can form, right?

Yes! When current passes through, you might see bubbles of oxygen or hydrogen. This is because electricity causes chemical reactions, known as electrolysis.

What kind of substances are involved in these reactions?

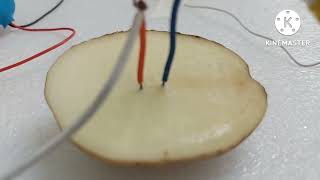
Great question! Typically, we use solutions of acids or salts. Does anyone remember the example we performed with the potato?

Sure! We observed chemical changes in the potato when we inserted the wires.

Exactly! That’s an excellent demonstration of chemical effects from electric current. Remember, electrolysis not only produces gases but alters the substances in the solution!

In summary, we learn that electric current through conducting solutions can lead to various chemical changes, including gas formation and color change.
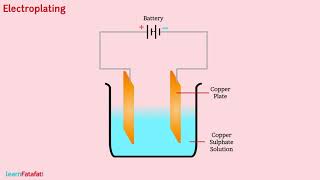
Electroplating
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s look at electroplating now. Who can tell me what that process involves?

It's when one metal is deposited onto another metal using electricity.

Exactly! Why is electroplating beneficial? Can someone share examples?

I’ve seen shiny bike parts or gold coatings on jewelry!

And it protects them from rusting, right?

Absolutely! Electroplating not only enhances appearance but also provides protection. Can anyone remember the fundamental principle behind it?

It's about transferring metal ions from one electrode to another while current flows!

Perfect! We can summarize our learning: Electroplating helps in decorating and protecting metal objects, ensuring durability and aesthetic appeal!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section discusses the conduction of electricity through liquids and the subsequent chemical reactions that occur when electric current flows through different solutions. It emphasizes hands-on activities to test various liquids, leading to an understanding of electrolysis, electroplating, and the distinction between good and poor conductors.
Detailed
In this section, we investigate the chemical effects of electric current, focusing on how different liquids can either conduct electricity or not. We learn that many liquids, especially solutions of acids, bases, and salts, are good conductors of electricity. Through practicality and experimentation, such as testing lemon juice and vinegar with a circuit tester, we observe that while some liquids allow the passage of electric current, others do not. Experiments demonstrate that the presence of ions, typically from dissolved substances, allows for electrical conduction.
Moreover, the section explores how an electric current can precipitate chemical reactions through electrolysis—producing gas bubbles and changing the properties of solutions. This leads us to the topic of electroplating, where a layer of one metal is deposited onto another through the process of applying current to a solution containing metal ions. Thus, electroplating has practical applications in various fields, making ordinary metal objects shiny or corrosion-resistant with metal coatings. Throughout, a series of activities and hypothetical questions lay the groundwork for understanding the principles governing these chemical effects.
Youtube Videos








Key Concepts
-
Chemical Effects of Electric Current: The phenomena that occur when an electric current passes through a conducting solution.
-
Conductivity: The ability of a substance to allow electrical currents to pass through it, often dependent on its ionic content.
-
Electrolysis: A chemical process that involves the passage of an electric current through a liquid or solution that conducts electricity.
-
Electroplating: A method to deposit a layer of metal onto another material using the electrical current.
Examples & Applications
Lemon juice and vinegar are good conductors of electricity due to their acidic content, which provides ions to facilitate current flow.
During electrolysis, passing current through water produces hydrogen and oxygen gas at the electrodes.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When liquids are sour, they'll conduct with power; acids and salts, making jumps, causing electrical hums.
Stories
Imagine a laboratory where a curious scientist tests various liquids. One day, lemon juice is the star, glowing bright under a small battery's power, teaching the scientist about conduction.
Memory Tools
Remember the acronym 'GASES' - for good conductors: Grape juice, Acids, Salts, Electrolytes, and Strong bases.
Acronyms
Use 'LEAD' - Liquids (L), Electrolysis (E), Acids (A), and Deposits (D) to remember electrolysis effects.
Flash Cards
Glossary
- Electrode
A conductor through which electricity enters or leaves an object, substance, or region.
- Electroplating
The process of depositing a layer of any desired metal on another material using electricity.
- Good Conductor
A material that allows electric current to flow through it easily.
- LED
Light Emitting Diode; a semiconductor light source that emits light when current flows through it.
- Poor Conductor
A material that does not allow electric current to flow through it easily.
Reference links
Supplementary resources to enhance your learning experience.
