Cement Chemistry
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Chemical Composition of Cement
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will start with the chemical composition of cement, particularly Ordinary Portland Cement, which is the most commonly used cement type. Who can tell me what materials are involved in making cement?

Is it made from limestone and clay?

That’s correct! When we calcine limestone and clay at high temperatures, we create clinker. What oxides do we have in cement, and what are their functions?

I remember some of them! Like CaO for strength gain.

And SiO₂ for creating strength-giving C-S-H!

Exactly! CaO, SiO₂, and others like Al₂O₃ and Fe₂O₃ contribute to the properties of cement. To remember them easily, think of 'C-S-H' for calcium-silicate-hydrate which is the key product responsible for strength. Can anyone explain what happens if there's too much lime?

It can cause unsoundness, right?

Well said! This concept fits with our understanding of material behavior in concrete. So remember, CaO supports strength but excess can lead to issues.
Bogue’s Compounds
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s dive into Bogue’s compounds. Can anyone name the four major compounds in cement?

C₃S, C₂S, C₃A, and C₄AF.

Correct! Each plays a unique role in hydration. Which compound acts quickly to contribute to early strength?

That would be C₃S!

It reacts slowly but adds strength later on.

Well summarized! The properties of these compounds illustrate how modifying cement chemistry can cater to different engineering needs. Remember, fast-reacting compounds like C₃A must be controlled with gypsum to prevent undesired rapid setting.
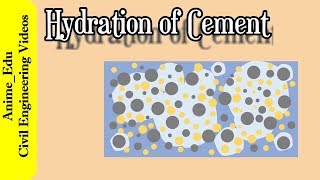
Hydration Process
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's talk about hydration. Who can explain what occurs during the hydration of cement?

Cement reacts with water and forms C-S-H gel and Ca(OH)₂.

Right! This process is vital for strength development. Can anyone tell me what role gypsum plays in this?

Gypsum slows down the hydration of C₃A, preventing flash setting.

Exactly! Managing setting time through the addition of gypsum is crucial in providing a practical work window for construction. Let’s not forget that high heat release is also generated during hydration!
Setting and Hardening
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

We’ll now differentiate between setting and hardening. Can anyone describe what setting means?

Setting is when the cement transitions from fluid to rigid state.

Great! And how is it different from hardening?

Hardening is when it gains strength over time.

Correct! Setting can take anywhere from 30 minutes to several hours, while hardening can continue for weeks. C-S-H formation is key here.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section delves into the chemical composition of Ordinary Portland Cement (OPC), detailing the major oxides and Bogue's compounds that influence the strength and setting properties. Key aspects of hydration reactions, the impact of gypsum, and the importance of controlling setting time are highlighted, along with insights into cement durability against chemical attacks.
Detailed
Cement Chemistry
Cement is a critical binding material in concrete, primarily identified as Ordinary Portland Cement (OPC). This section elaborates on the chemical factors that govern the performance of concrete, underscoring the significance of cement chemistry in engineering applications.
2.1 Chemical Composition of Cement
The primary ingredients of OPC—Lime (CaO), Silica (SiO₂), Alumina (Al₂O₃), Iron Oxide (Fe₂O₃), Magnesia (MgO), Sulphur Trioxide (SO₃), and Alkali Metals (Na₂O, K₂O)—play crucial roles in determining the strength and durability of concrete. For instance, while CaO promotes strength gain, excess can lead to unsoundness.
2.2 Major Compounds (Bogue’s Compounds)
Cement contains four major compounds identified through Bogue’s equations: Tricalcium Silicate (C₃S), Dicalcium Silicate (C₂S), Tricalcium Aluminate (C₃A), and Tetracalcium Aluminoferrite (C₄AF). Each compound plays distinct roles in the hydration process and in establishing concrete properties.
2.3–2.5 Hydration Process
Hydration is the exothermic reaction where cement interacts with water, releasing heat, which is vital for hardening. Gypsum is added to manage setting times and control C₃A reactions, preventing flash sets that could compromise concrete workability.
2.6–2.10 Setting and Hardening
The transition from fluid to solid occurs during the setting phase, influenced strongly by the chemical composition of cement. Long-term strength development occurs as hydration continues over weeks and months, where the formation of C-S-H gel is key.
Unsafe conditions may arise from unsoundness due to free lime and excessive sulphates, while the alkali-silica reaction can lead to cracking. Various cement types, including rapid hardening and sulphate-resisting cement, are tailored to specific engineering needs.
In conclusion, understanding the complex relationship between the chemical composition and the resulting properties of cement is paramount for optimizing concrete performance.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Cement Chemistry
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Cement is the binding material that holds concrete together. Understanding cement chemistry is crucial for civil engineers, as it governs the strength, durability, setting time, and performance of concrete. This chapter delves into the chemical composition of cement, its hydration process, the role of various compounds, and how modifications in chemistry can tailor the cement to specific engineering needs.
Detailed Explanation
Cement is a critical material used in the construction of concrete, which is a composite material comprising cement, aggregates (like sand and gravel), and water. The chemistry behind cement is vital for engineers to ensure that concrete performs well over its lifespan. This introduction sets the stage by highlighting the importance of understanding the chemical properties and processes in cement to enhance the quality and reliability of concrete structures.
Examples & Analogies
Think of cement as the glue that holds together the different elements of a sandwich. Just like how the right type of glue makes sure the sandwich stays intact, the proper chemistry of cement ensures that concrete can bear the necessary loads and withstand environmental conditions.
Chemical Composition of Cement
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Ordinary Portland Cement (OPC), the most commonly used cement in concrete technology, is manufactured by calcining a mixture of calcareous and argillaceous materials at high temperatures (around 1450°C). The resulting product is called clinker, which is then ground with a small amount of gypsum.
Detailed Explanation
Ordinary Portland Cement (OPC) is created through a high-temperature process that involves raw materials rich in calcium and clay-like materials. This heating causes chemical reactions that form clinker, the primary substance in cement, which is then finely ground and mixed with gypsum. This process transforms raw materials into a highly effective binding agent for concrete.
Examples & Analogies
Manufacturing cement can be compared to baking cookies. Just as you mix flour (the main ingredient), sugar, and eggs (other ingredients) and then bake them to transform them into cookies, the real ingredients for cement are heated to create clinker and then ground, similar to how you would ground your cookie mixture to be ready for baking.
Major Oxides Present in Cement
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Major Oxides Present:
- Lime CaO 60–67
- Silica SiO₂ 17–25
- Alumina Al₂O₃ 3–8
- Iron oxide Fe₂O₃ 0.5–6
- Magnesia MgO 0.1–4
- Sulphur trioxide SO₃ 1–3
- Alkalis (Na₂O, K₂O) - 0.2–1.3
Functions of Oxides:
- CaO (Lime): Responsible for strength gain. Excess can cause unsoundness.
- SiO₂ (Silica): Combines with CaO to form strength-giving C-S-H.
- Al₂O₃ (Alumina): Aids in quick setting and reduces clinkering temperature.
- Fe₂O₃ (Iron oxide): Adds hardness and contributes to color.
- MgO: Small amounts add strength; excess causes expansion.
- SO₃ (Sulphur trioxide): Controls setting time.
- Alkalis (Na₂O, K₂O): Affect durability and may cause efflorescence.
Detailed Explanation
Cement contains various oxides, each contributing specific properties crucial for the overall performance of concrete. For example, lime (CaO) is vital for strength development, while silica (SiO₂) is essential for the formation of calcium silicate hydrate (C-S-H), which is the main bonding agent in concrete. Understanding the roles of these oxides helps in tailoring cement for different applications and ensuring high performance.
Examples & Analogies
Think of these oxides as ingredients in a recipe for a cake. Just like how flour contributes to the cake's structure, sugar provides sweetness, and eggs bind everything together, each oxide in cement contributes to its properties and performance, affecting how the concrete ultimately behaves.
Major Compounds in Cement (Bogue's Compounds)
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Using the oxide composition, four main compounds in cement can be identified through Bogue’s equations:
- Tricalcium silicate 3CaO·SiO₂ C₃S 40–60%
- Dicalcium silicate 2CaO·SiO₂ C₂S 15–35%
- Tricalcium aluminate 3CaO·Al₂O₃ C₃A 5–12%
- Tetracalcium aluminoferrite 4CaO·Al₂O₃·Fe₂O₃ C₄AF 6–10%
Role of Major Compounds:
- C₃S (Tricalcium silicate): Fast-reacting, contributes to early strength (1–7 days).
- C₂S (Dicalcium silicate): Reacts slowly, contributes to strength after 7 days and improves durability.
- C₃A (Tricalcium aluminate): Highly reactive, controls setting time. Responsible for initial flash set (controlled using gypsum). Most vulnerable to sulphate attack.
- C₄AF (Tetracalcium aluminoferrite): Minor contribution to strength. Reduces clinkering temperature. Imparts grey color to cement.
Detailed Explanation
The four major compounds identified in cement through Bogue's equations each serve critical roles in the properties of cement. C₃S is essential for initial strength gain, while C₂S contributes to long-term strength. C₃A is important for regulating how quickly cement sets, and C₄AF has minor contributions but assists in manufacturing. Ultimately, knowing these compounds can guide engineers in selecting the right type of cement for their projects.
Examples & Analogies
You can think of these compound roles like the various instruments in a symphony orchestra. The violin (C₃S) plays a key theme (strength), while the piano (C₂S) provides harmony (long-term durability), and the percussion (C₃A) keeps the tempo (setting time). Each instrument (or compound) is important for the overall performance (quality) of the music (concrete).
Key Concepts
-
Chemical Composition of Cement: The main oxides and their functions in influencing strength and setting time.
-
Bogue’s Compounds: The primary compounds in cement responsible for various hydration behaviors and properties.
-
Hydration: The reaction of cement with water leads to hardening and strength development.
-
Setting Time: The critical duration for transitioning from a fluid to a solid state in concrete.
Examples & Applications
Ordinary Portland Cement contains various oxides like CaO and SiO₂, which influence the setting and strength of concrete.
In mass concrete structures like dams, low heat cement is used to minimize hydration heat, preventing cracking.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Cement sets and gains its might, with C-S-H it’ll reach new height!
Stories
Imagine a race against time where C₃A is like a sprinter who overexerts quickly, causing problems unless gypsum, a wise coach, steps in to teach it patience.
Memory Tools
Remember 'Cements at Bogue's Factory': C-S, C-A, C-2 for understanding cement compounds!
Acronyms
Use 'HAP' - Hydration, Aluminate (C₃A), Portland Cement to recall key cement hydration concepts.
Flash Cards
Glossary
- CSH (Calcium Silicate Hydrate)
The gel-like product formed during the hydration of cement that contributes to the strength of concrete.
- Gypsum
A mineral added to cement to control setting time and manage hydration reactions.
- Hydration
The chemical reaction between cement and water that results in hardening.
- Bogue's Compounds
The four primary compounds identified in cement that influence its properties—tricalcium silicate (C₃S), dicalcium silicate (C₂S), tricalcium aluminate (C₃A), and tetracalcium aluminoferrite (C₄AF).
- Setting Time
The progression of time that it takes for cement to transition from a semi-fluid state to a rigid state.
Reference links
Supplementary resources to enhance your learning experience.
