Hydration Reactions
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Hydration of C₃S and C₂S
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're focusing on the hydration reactions of Tricalcium Silicate, or C₃S, and Dicalcium Silicate, C₂S. Who can tell me what happens when C₃S reacts with water?

It forms calcium silicate hydrate and calcium hydroxide, right?

Exactly! The reaction is: C₃S + H₂O → C-S-H + Ca(OH)₂ + Heat. Can someone explain why C-S-H is important?

C-S-H is responsible for the strength of the concrete.

That's correct! And what about C₂S? How is its reaction different?

It also forms C-S-H but generates less heat compared to C₃S.

Well done! So, C₂S contributes to strength over a longer period—important for durability.

To remember these reactions, think 'C-S-H is crucial for strength'. Let's summarize: C₃S gives early strength due to its heat generation!
Hydration of C₃A and Gypsum's Role
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let's discuss the hydration process of Tricalcium Aluminate, or C₃A. Why is the addition of gypsum significant?

Gypsum slows down the rapid reaction of C₃A to prevent flash setting.

Exactly! Without gypsum, the reaction C₃A + H₂O occurs too quickly. Can anyone write down the products formed?

It forms ettringite and heat.

Yes! The complete reaction is: C₃A + Gypsum + Water → Ettringite. As gypsum gets depleted, ettringite can transform into monosulphate. Understanding this helps inform the use of additives!

What happens if we add too much gypsum?

Great question! Too much can delay setting too much and affect workability. So balance is key!

Let's recap: Gypsum controls C₃A hydration speed, ensuring proper setting!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In hydration reactions, major cement compounds like C₃S and C₂S interact with water to produce calcium silicate hydrate (C-S-H) and calcium hydroxide, essential for increasing concrete strength. The hydration of C₃A involves more complex reactions requiring gypsum to control setting time.
Detailed
Hydration Reactions
Hydration reactions represent crucial chemical reactions involving cement compounds and water. The primary compounds involved in hydration are Tricalcium Silicate (C₃S) and Dicalcium Silicate (C₂S). When C₃S reacts with water (H₂O), it produces Calcium Silicate Hydrate (C-S-H), which is the main component providing strength to concrete, as well as Calcium Hydroxide (Ca(OH)₂), which increases the pH but can make concrete susceptible to chemical attacks. The reactions can be summarized as follows:
- C₃S + H₂O → C-S-H (gel) + Ca(OH)₂ + Heat
- C₂S + H₂O → C-S-H (gel) + Ca(OH)₂ (less) + Less Heat
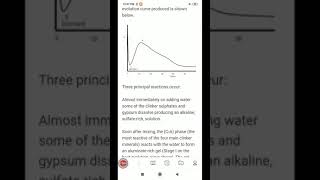
On the other hand, Tricalcium Aluminate (C₃A) undergoes a very rapid hydration process. To manage this fast reaction and prevent an immediate setting (flash set), gypsum is added. The reaction can be represented as:
- C₃A + Gypsum + Water → Ettringite (C₆AŜ₃H₃₂)
As the gypsum is consumed, ettringite can further transform into Monosulphate:
- Ettringite + C₃A → Monosulphate (C₄AŜH₁₂)
These hydration reactions not only contribute to the immediate strength of concrete but also affect its long-term durability and resistance to environmental conditions.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
C₃S and C₂S Hydration
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
These compounds react with water to form:
- Calcium silicate hydrate (C-S-H): Responsible for strength.
- Calcium hydroxide (Ca(OH)₂): Improves pH but makes concrete vulnerable to chemical attack.
Reactions:
- C₃S + H₂O → C-S-H (gel) + Ca(OH)₂ + Heat
- C₂S + H₂O → C-S-H (gel) + Ca(OH)₂ (less) + Less Heat
Detailed Explanation
The hydration of two key compounds in cement, Tricalcium Silicate (C₃S) and Dicalcium Silicate (C₂S), occurs when they come into contact with water. These reactions produce Calcium Silicate Hydrate (C-S-H), which is crucial for the strength of concrete, and Calcium Hydroxide (Ca(OH)₂), which increases the pH level. While the formation of C-S-H is beneficial as it contributes to strength, Ca(OH)₂ can make concrete more susceptible to certain chemical attacks. The first reaction involving C₃S is exothermic, meaning it generates heat, while the second reaction with C₂S produces less heat and less Ca(OH)₂. These reactions illustrate how the types of compounds in cement can influence both the setting and long-term behavior of concrete.
Examples & Analogies
Think of C₃S and C₂S like two different types of slow-release fertilizers. When water (think of it as the planting environment) interacts with these fertilizers, it gradually releases nutrients (C-S-H) that help plants grow strong. However, too much of a certain nutrient (Calcium Hydroxide) may not be beneficial and can attract pests (chemical attacks on concrete). Just like in gardening, it’s essential to balance the right inputs to ensure strong, durable concrete.
C₃A Hydration
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Very fast reaction; gypsum is added to slow it down and prevent flash set.
- Forms ettringite (a stable product):
- C₃A + Gypsum + Water → Ettringite (C₆AŜ₃H₃₂)
As gypsum is consumed, ettringite can convert to monosulphate:
- Ettringite + C₃A → Monosulphate (C₄AŜH₁₂)
Detailed Explanation
The hydration of Tricalcium Aluminate (C₃A) occurs very quickly when water is added. To manage this rapid reaction, gypsum is incorporated, which helps to slow down the process and prevents a phenomenon known as flash setting, where concrete hardens too quickly to be useful. When C₃A reacts with gypsum and water, it forms a stable compound called ettringite, which is vital for the early strength of concrete. Over time, as gypsum is depleted, ettringite can change into another compound called monosulphate, which plays a role in maintaining the integrity of the hardened cement paste.
Examples & Analogies
Imagine making a cake, where you need to control how quickly the batter bakes. If you add too much heat too quickly (like C₃A hydration without gypsum), your cake might rise too fast and collapse (flash set). Gypsum acts like a baking sheet that controls the heat, allowing the cake to rise evenly (stable formation of ettringite), ensuring it turns out just right!
Key Concepts
-
Hydration Reactions: The process where cement reacts with water to form hydrates that provide strength and stability.
-
Calcium Silicate Hydrate (C-S-H): A gel formed from the hydration of cement that is a primary contributor to concrete strength.
-
Role of Gypsum: Added to slow down the hydration of C₃A to prevent flash setting.
Examples & Applications
When mixing concrete, adding water initiates hydration reactions, leading to the production of C-S-H, which solidifies and strengthens the mixture.
Gypsum is commonly added in a percentage of around 3-5% by weight of the cement to control the setting time effectively.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When C₃S meets H₂O, C-S-H starts to grow!
Stories
Imagine a magician named C₃S who, when splashed with water, transforms into a gel (C-S-H) that makes concrete strong!
Memory Tools
C-S-H stands for Calcium Silicate Hydrate and holds the key to concrete’s might.
Acronyms
CCH - 'Cement + C₃A + Hydration' to remember essential components in hydration reactions.
Flash Cards
Glossary
- Hydration
The chemical reaction between cement compounds and water that leads to the hardening of concrete.
- CSH
Calcium Silicate Hydrate, a gel that forms during the hydration process and is responsible for the strength of concrete.
- Ettringite
A stable compound formed during the hydration of C₃A in the presence of gypsum.
- Monosulphate
A compound formed from ettringite when gypsum is consumed during hydration.
- Flash Set
An immediate setting of cement that can occur due to rapid hydration reactions.
Reference links
Supplementary resources to enhance your learning experience.
