Heat of Hydration
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Heat of Hydration
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’re exploring the heat of hydration, which refers to the heat released when cement hydrates. Can anyone tell me why this process is essential in concrete technology?

I think it helps in the setting process of concrete.

Exactly! The released heat aids in the hydration process, making concrete set faster. Let's look closer at the compounds and their heat outputs.

Which compound releases the most heat?

That would be C₃A, it has the highest heat of hydration at 207 cal/g, important for quick setting. But why might that be a problem?

Too much heat can cause cracks, right?

Yes! Especially in large structures like dams where rapid temperature changes can lead to thermal cracking. Remember, hydration is exothermic!
Cement Compounds and Heat Output
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s break down how much heat each cement compound releases. C₃S produces 120 cal/g, while C₂S contributes 62 cal/g. Why do you think there's such variation?

Maybe some compounds react more quickly with water?

Correct! C₃S reacts faster, which is why it’s crucial for achieving early strength. It balances the slower-reacting C₂S that contributes to strength later. Can someone summarize these for me?

C₃A is fast and hot, C₃S is also hot but not as much, and C₂S is slow and cool.

Great summary! Remember this heat balance helps engineers tailor properties of concrete for different environments.
Practical Implications of Heat of Hydration
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss practical implications. Why would understanding heat of hydration matter in cold or hot climates?

In cold weather, we want to retain heat to help set the concrete, right?

Exactly! We can use this heat beneficially. However, what about in mass concrete applications?

The heat can cause thermal cracking because of temperature gradients.

Spot on! This is why engineers carefully design mixes for mass concrete, controlling heat production to avoid damage.

So, it’s all about balance!

That's right! Balance is key in managing hydration and its effects.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section explains the concept of heat of hydration, detailing the heat generated by different cement compounds as they hydrate. It highlights the importance of this heat in setting and hardening concrete, particularly in various environmental conditions, and the potential issues it can cause in mass concrete applications.
Detailed
Heat of Hydration
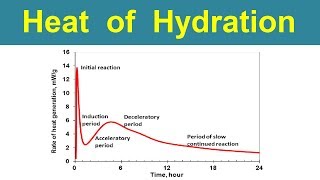
Hydration is the critical chemical reaction that takes place when cement compounds come in contact with water, resulting in an exothermic process that produces heat, known as the heat of hydration. Each type of cement compound releases different amounts of heat:
- C₃S (Tricalcium Silicate) - Releases approximately 120 cal/g.
- C₂S (Dicalcium Silicate) - Releases about 62 cal/g.
- C₃A (Tricalcium Aluminate) - Produces significant heat, totaling 207 cal/g.
- C₄AF (Tetracalcium Aluminoferrite) - Yielding around 100 cal/g.
Importance of the Heat of Hydration
The heat generated during hydration is vital as it aids in the setting and hardening of concrete. In colder climates, the additional heat can be beneficial for adequate curing. However, in the case of mass concrete structures, such as dams, the excessive heat released can lead to thermal cracking due to rapid temperature changes. Understanding the balance of heat produced by different compounds is crucial for effective cement mix design.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Hydration Process
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Hydration is the chemical reaction between cement compounds and water. This process is exothermic, releasing heat, known as the heat of hydration.
Detailed Explanation
The hydration process begins when cement is mixed with water. During this interaction, a chemical reaction occurs, leading to the hardening of the cement. Importantly, this reaction is exothermic, meaning it generates heat. This heat is essential, as it contributes to the setting and hardening phases of concrete, making it stronger over time.
Examples & Analogies
Think of hydration in concrete like baking bread. When the dough (cement) is mixed with water, it begins a chemical reaction that creates heat and makes the dough rise (harden). Just as the heat in the oven helps the bread become firmer, the heat from hydration helps cement set and gain strength.
Typical Heat Released by Compounds
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Typical Heat Released by Compounds:
| Compound | Heat of Hydration (cal/g) |
|---|---|
| C₃S | 120 |
| C₂S | 62 |
| C₃A | 207 |
| C₄AF | 100 |
Detailed Explanation
Different compounds within cement release varying amounts of heat during the hydration process. For example, Tricalcium Silicate (C₃S) releases 120 calories per gram, while Tricalcium Aluminate (C₃A) releases a higher 207 calories per gram. This variance is crucial because it allows engineers to predict how much heat will be generated during the hydration process, which affects the concrete's performance and temperature management.
Examples & Analogies
Imagine you are cooking different types of dishes. Some, like a stir-fry (C₃A), cook quickly and generate a lot of heat, while others, like a slow-cooking stew (C₂S), generate heat over time but at a much slower pace. Understanding how much heat each dish (compound) will produce helps you plan your cooking (concrete setting).
Importance of Heat of Hydration
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Importance:
- Helps in the setting and hardening of concrete.
- High heat is beneficial in cold weather.
- In mass concrete structures (e.g., dams), excessive heat can cause cracking.
Detailed Explanation
The heat produced during hydration plays a critical role in how concrete sets and hardens. In cold weather, this heat can be beneficial, aiding in the setting process at lower temperatures. However, in large structures, such as dams, if too much heat is generated, it can lead to thermal cracking. Thus, managing the heat of hydration is essential for the overall integrity of concrete structures.
Examples & Analogies
Picture yourself building a snowman on a cold day. The warmth from your hands (heat of hydration) helps to shape the snow. If it gets too warm, parts of the snowman might melt or crack under pressure. Similarly, in concrete construction, controlling the heat balance ensures the concrete maintains its strength and stability without risking damage.
Key Concepts
-
Hydration releases heat: The chemical reaction of hydration between water and cement compounds releases heat.
-
C₃A produces the most heat: It's important but can cause problems in large structures.
-
Temperature effects: The heat generated has implications in cold weather settings and for mass concrete applications.
Examples & Applications
In cold weather, the heat from hydration helps cure concrete effectively, preventing freeze damage.
In mass concrete, excessive heat can cause thermal cracking, therefore heat management is essential in design.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When cement meets water, it releases heat, hydration is its only treat!
Stories
Imagine mixing cement and water on a chilly day. The warmth generated from their union helps the concrete to set better, just like a warm blanket on a cold night.
Memory Tools
HAVE - Heat Aids in Value of Early strength.
Acronyms
H.E.A.T - Hydration == Exothermic, Aids, Temperature management.
Flash Cards
Glossary
- Heat of Hydration
The exothermic heat produced when cement compounds react with water during hydration.
- C₃S
Tricalcium Silicate, a major compound in cement that contributes to early strength.
- C₂S
Dicalcium Silicate, a cement compound that contributes to long-term strength.
- C₃A
Tricalcium Aluminate, known for its rapid reaction and significant heat release.
- C₄AF
Tetracalcium Aluminoferrite, which contributes minor strength but plays other roles in cement.
Reference links
Supplementary resources to enhance your learning experience.
