Environmental Quality Monitoring Analysis
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Mass Concentration
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to understand mass concentration, which is a fundamental concept in environmental quality monitoring. We denote it by Rho. Can anyone tell me what Rho represents?

Is it the density of a substance?

Exactly! Rho stands for mass per unit volume. For example, Rho A1 refers to the concentration of substance A in air. What about Rho A2?

That would be the concentration of A in water.

Correct! Remember that understanding these concepts helps us know how substances can interact in different environments. Remember 'Rho' for relating to various media like air and water.

Why don’t we always use just volume for concentration?

Good question! Mass concentration is more relevant in environmental contexts since we're often dealing with solid forms and heterogeneous mixtures. It’s a lot more practical.
Aqueous Solubility and Vapour Pressure
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's talk about aqueous solubility. It’s represented by Rho A2*. Can anyone explain what the asterisk signifies?

Does it mean it’s in equilibrium?

Precisely! It indicates that we are discussing equilibrium conditions. A substance’s ability to dissolve in water is vital for evaluating its environmental impact. Can anyone relate vapour pressure to this concept?

It's Rho A1*, showing concentration in the air?

Correct! Understanding both properties helps us assess how chemicals behave in water and air. So, remember: 'Aqueous solubility' relates to water, while 'vapour pressure' is all about air.
Henry's Constant and Partitioning
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next up, we have Henry's Constant, which illustrates the relationship between concentrations in two phases. Who remembers what that means?

It’s the ratio of a substance's concentration in air to its concentration in water?

Exactly! So when we discuss how chemicals distribute themselves in environments, we also look at partitioning—like partition constant KA32 for water and solids. Why do you think it's important to understand this?

It shows how easily a chemical can move into different environments, right?

Spot on! This helps us predict potential contamination and helps in remediation strategies. Always think of 'partitioning' when assessing chemical behavior in environmental contexts.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, the primary focus is on understanding different types of mass concentration represented by Rho, aqueous solubility, partition constants, and how these factors play a role in environmental quality monitoring. The content elaborates on the importance of analyzing the concentration difference among various phases like air, water, and solid to assess environmental impacts.
Detailed
Environmental Quality Monitoring Analysis
This section delves into the principles and terminology involved in environmental quality monitoring, specifically through the analysis of concentration variations in different media. The key focus is on mass concentration denoted by the symbol Rho, where different indices correspond to various phases:
- A1: Air,
- A2: Water,
- A3: Solid,
- A4: Pure Chemical.
The section further discusses important properties such as:
1. Aqueous Solubility: Represented as Rho A2, showing the equilibrium concentration of a substance in water.
2. Vapour Pressure: Denoted by Rho A1, indicating equilibrium concentration in air.
3. Henry’s Constant: A ratio often used to define the relationship between concentrations in different phases, critical for understanding substances in environmental systems.
4. Partitioning: The process of a chemical's distribution between water and solids, represented by partition constants such as KA32. This is crucial for understanding contamination processes in soils and groundwater.
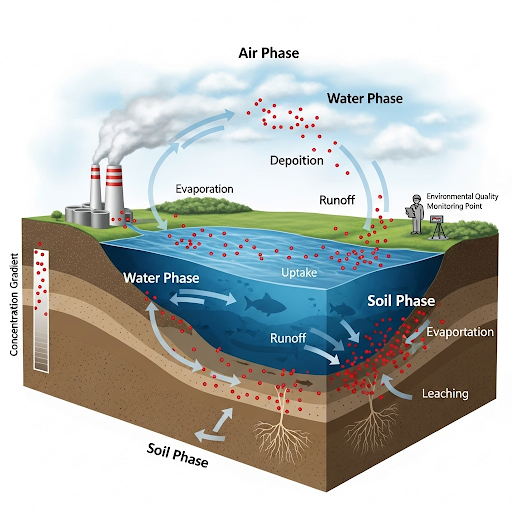
Overall, the nuances in concentration—identified through Rho and its applications—are critical for accurately assessing environmental effects and guiding remediation efforts.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Mass Concentration Overview
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Our main quantity of interest is concentration. And we are looking at mass concentration. So the mass concentration symbol is Rho, so Rho of A in some medium (air, water, etc.). I have indices for I (i) to indicate air (1), water (2), solid (3), and pure chemical (4).
Detailed Explanation
In this chunk, we're introduced to the concept of mass concentration, which is central to environmental monitoring. The symbol Rho (ρ) represents mass concentration, specifically referring to the amount of mass of a substance (A) per unit volume in different media such as air (Rho A1), water (Rho A2), and solid (Rho A3). The use of indices helps differentiate the medium being referred to—whether it’s air, water, or solid. For instance, Rho A1 is the mass concentration in air, while Rho A2 is for water.
Examples & Analogies
Consider a glass of water mixed with sugar. The concentration of sugar in the water can be denoted by Rho A2, which means there's a specific amount of sugar per volume of water. If we were to measure how much sugar is in the air above the glass, that would be Rho A1, showing how substances can exist in multiple phases.
Concentration Measurement Challenges
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In soil, Rho A3 indicates the concentration of A on solid but measuring solid volume is tricky due to its porous and heterogeneous nature. Thus, we often use mass fraction (wA3), which represents the mass of A divided by the mass of the solid.
Detailed Explanation
The chunk highlights the difficulty of measuring concentration in soil due to its complex structure. Soil is not uniform; it consists of particles of varying sizes and porosity, making it hard to accurately measure the volume of soil. Instead, scientists use mass fraction (wA3), which is simply the mass of the substance A divided by the mass of the solid material itself. This approach avoids the complications involved in determining the precise volume.
Examples & Analogies
Imagine trying to measure the exact volume of a handful of sand. It's not straightforward because the grains vary in size and the spaces between them are filled with air. Instead, if you weighed the sand and then quantified how much of it is made up of a specific color of sand (say, red sand), this gives a clear indication of content without needing to measure complex volumes.
Equilibrium Concentrations and Properties
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
We apply this nomenclature to three physical properties—aqueous solubility (Rho A2), vapor pressure (Rho A1), and Henry's constant, which is the partitioning constant between two phases of A.
Detailed Explanation
This chunk focuses on three critical physical properties: aqueous solubility, vapor pressure, and Henry's constant. Aqueous solubility indicates how much of substance A can dissolve in water at a certain temperature, denoted as Rho A2. Vapor pressure reflects the concentration of A in the air (Rho A1) when it's in equilibrium with its liquid phase. Henry's constant, meanwhile, represents how much of A partitions between water and air, providing insights into its behavior in different environments.
Examples & Analogies
When you open a can of soda, the carbon dioxide (CO2) gas escapes into the air. The amount of CO2 that remains dissolved in the liquid is its aqueous solubility, while the gas in the air is determined by its vapor pressure. Henry's Law can help predict how much will escape into the air, showing the balance between what's in the can and what's in the surrounding atmosphere.
Partitioning Between Phases
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The partitioning of a chemical A between water and solids (KA32) is critical, often represented as WA3/Rho A2 at equilibrium. It measures how chemicals move from water into solids.
Detailed Explanation
Here, partitioning describes how a chemical, like A, distributes itself between two phases—water and solids. The equation KA32 = WA3/Rho A2 at equilibrium represents this relationship, where WA3 is the mass fraction of A on the solid and Rho A2 is the concentration of A in water. Understanding partitioning is essential for predicting how pollutants travel through the environment and how they may affect soil and groundwater.
Examples & Analogies
Think about pouring dye into a bowl of water that contains pebbles. Initially, the dye is predominantly in the water, but over time, some of it will adhere to the surface of the pebbles, creating a balance between what remains in the water and what bonds with the pebbles. This equilibrium helps understand how pollutants might behave in real-world scenarios.
Implications of Partitioning
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This partitioning effect becomes crucial when a contaminant is added to soil; it can travel down to groundwater, affecting clean water sources.
Detailed Explanation
This chunk stresses the real-world implications of how chemicals migrate in the environment. When contaminants are introduced into soil, they can move downwards due to gravity and other forces, reaching the groundwater. This process raises concerns for water safety and environmental health because once a contaminant reaches groundwater, it can lead to broader ecosystem impacts.
Examples & Analogies
Consider a situation where a company improperly disposes of chemicals, causing them to leach through soil layers and potentially polluting a nearby well that serves as a drinking water source. Understanding the partitioning process helps regulators create policies to prevent such contamination and ensures safer water supplies for communities.
Key Concepts
-
Mass Concentration: The amount of substance in relation to the volume of air, water, or solid.
-
Aqueous Solubility: The ability of a substance to dissolve in water at equilibrium.
-
Vapour Pressure: The equilibrium pressure of vapor in contact with its liquid.
-
Henry's Constant: A ratio defining the concentration of a substance in air relative to water.
-
Partitioning: The behavior of substances as they distribute themselves between water and solid phases.
Examples & Applications
Example of Rho A1: Concentration of pollutants in the air measured to assess air quality.
Example of partitioning: How oil may separate from water due to its lower density, impacting environmental cleanup strategies.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Rho in the air, and Rho in the sea, helps us see, how substances can be free.
Stories
Imagine a fish swimming in a lake. The pollutants from air dissolve into the water. The fish's habitat is at risk, just like air and water interact and affect each other.
Memory Tools
Remember Rho A1, A2, and A3 for air, water, and solids; they’re the key to understanding quality!
Acronyms
RAP
Rho for Air
Rho for water
and Partition constants help us monitor.
Flash Cards
Glossary
- Mass Concentration
The mass of a substance divided by the volume of the medium, denoted by Rho.
- Aqueous Solubility
The concentration of a compound dissolved in water, often represented with a star notation to denote equilibrium.
- Vapour Pressure
The pressure exerted by a vapor in equilibrium with its liquid or solid form, indicated by Rho A1*.
- Henry's Constant
A coefficient representing the ratio of a substance's concentrations in two different phases at equilibrium.
- Partitioning
The distribution of a chemical compound between two phases, such as solid and liquid.
Reference links
Supplementary resources to enhance your learning experience.
