Mass Fraction and Dimensions
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Mass Concentration
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will start with mass concentration, which we denote as Rho (ρ). Can anyone tell me what mass concentration refers to?

Is it the mass of a substance per unit volume?

Exactly! Rho is calculated as mass divided by volume, typically expressed in kg/m³. This is crucial for our studies. Any questions on that so far?

How do we express concentration in air versus water?

Great question! We use Rho A1 for air and Rho A2 for water. Remember these designations as they will be vital in our examples. Now let’s look at...
Understanding Mass Fraction
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Moving on, mass fraction is denoted using ‘w’. Can someone explain what mass fraction represents?

I think it’s the mass of the substance divided by the mass of the solid?

That's right! We often encounter problems where the volume of solids isn't straightforward to evaluate, so we use mass fraction for convenience. Good recall!

And can Rho be used for solids as well?

Yes, we can express concentration for solid in terms of Rho as well but substituting mass with mass of the solid. Keep in mind, all these terms are vital for our next topic on partition constants.
Exploring Partition Constants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s talk about partition constants. They describe the equilibrium distribution of a chemical between different phases. Can anyone give me an example?

Is it the ratio of concentration in air to that in water?

Absolutely! This is precisely how we express K A21 for air and water. Remember, equilibrium is crucial here, so we denote it with a star. Why is equilibrium important?

Because it shows the balance of concentrations in different phases?

Exactly! Understanding this balance helps us comprehend how contaminants move through the environment.
Mass Concentration in Soil
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s discuss soil. When we speak about mass concentration in soil, we often find it difficult to measure volume. What method can we use instead?

We can use the mass of the solid instead!

Exactly! We represent it as M A over M3, which brings us back to our mass fraction. This approach simplifies analysis of soil contaminants.

How does this relate to environmental quality monitoring?

This understanding allows us to assess the fate of various chemicals as they traverse through soil and waters. It’s vital for effective monitoring!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section explains mass concentration (denoted as Rho), mass fraction, and their relevance in environmental monitoring. It introduces different terms for concentration in various media and discusses partition constants, particularly in the context of chemical distribution between air, water, and solids.
Detailed
Mass Concentration and Fraction
This section elaborates on the concepts of mass concentration, symbolized as Rho (ρ), representing mass per unit volume (mass/volume), typically in units such as kg/m³. The key focus is on mass concentration across different media like air (Rho A1), water (Rho A2), and solids (Rho A3). Mass fraction, symbolized as ‘w’ or ‘Ω’, is defined as the mass of a substance divided by the mass of solid, especially when the volume of solids is hard to ascertain due to their heterogeneous structure.
Partition Constants
The section also defines partition constants, which describe the equilibrium distribution of chemicals between phases, specifically air, water, and solids. The general ratio for partition constants, such as the water-solid system (K A32), is introduced. This aspect highlights the chemical's behavior in various environments and their significance in environmental monitoring.
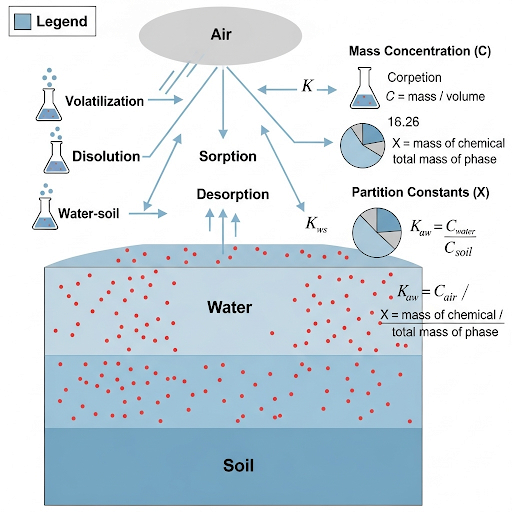
By developing an understanding of these terms, practitioners can better assess environmental quality and the movement of substances within different media.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Mass Concentration
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So the mass concentration symbol is Rho, so Rho of A and in some medium. Our main quantity of interest is concentration, and we are looking at mass concentration.
Detailed Explanation
Mass concentration is a fundamental concept in chemistry that refers to the mass of a substance (A) in a specific volume of a medium (like air or water). This is represented by the symbol Rho (ρ). For example, if we have a concentration of a chemical in water, it would be expressed as Rho A2, meaning the mass concentration of A in water.
Examples & Analogies
Imagine a glass of water with sugar dissolved in it. The amount of sugar per volume of water is the mass concentration. If you add more sugar, the concentration increases, similar to how Rho changes with the mass of a chemical.
Nomenclature for Different Mediums
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
I have indices for I have to put some number here to indicate whether something here to indicate it with air or water. So here, I have 'i', 'i' equals 1 is 'air', 'i' equals 2 is 'water', '3' corresponds to 'solid', '4' corresponds to 'pure chemical'.
Detailed Explanation
In this nomenclature system, different indices indicate in which medium a chemical is found. For example, 'Rho A1' represents the mass concentration of a chemical in air, while 'Rho A2' represents it in water, and 'Rho A3' is for solids like soil. This helps clarify the environment in which we're measuring concentrations.
Examples & Analogies
Consider a laboratory where scientists are testing a new drug. Different tests may involve the drug's concentration in air, water, and its solid form. The use of different indices allows them to keep track of where the drug is, making their findings clearer.
Mass Fraction Concept
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
We do a M A over M3. It is the mass of the solid itself; mass fraction symbol is ‘w’ or ‘Omega’. This is also called 'L oading', 'WA3'.
Detailed Explanation
The mass fraction (w or Ω) is defined as the mass of a chemical (A) divided by the mass of the solid (M3). This fraction helps us understand how much of the solid's mass is made up of the chemical. Unlike measuring concentration in liquid, this measurement is often more straightforward because it relies only on the mass of the solids involved.
Examples & Analogies
Think of baking a cake. If you have a cake mix (solid) and you measure how much cocoa powder (chemical) you add to it, the mass fraction tells you how much of your cake mix is made up of cocoa. If you put in more cocoa, the fraction increases, which can change the taste and color of your cake.
Equilibrium Properties
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When we reference properties like aqueous solubility or vapor pressure, we use a star notation to indicate that they are equilibrium properties.
Detailed Explanation
The star notation (e.g., Rho A2*) signifies that the values discussed are in a state of equilibrium. This means the concentration of a substance has reached a steady state where the rate of entering and leaving the medium (e.g., liquid or gas) are equal. Understanding equilibrium is crucial for predicting how chemicals behave in different environments.
Examples & Analogies
Consider a saturated solution of salt in water. When you keep adding salt, it dissolves until no more can dissolve. At this point, the system is in equilibrium: the rate of salt dissolving equals the rate of salt crystallizing out of the solution.
Partition Constants
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This is a partition constant or distribution constant, typically denoted as K. It describes the distribution of a chemical between two phases.
Detailed Explanation
A partition constant (K) expresses how a chemical distributes itself between two different media—in this case, air and water (Rho A1 and Rho A2). The equilibrium concentration ratio of the chemical in each medium at equilibrium can be represented by this constant. Understanding these constants is crucial for environmental and chemical engineers to predict how substances behave when they move between phases.
Examples & Analogies
Imagine a sponge soaked in water. The water inside the sponge represents the concentration in one phase, while the air around it represents the second phase. The partition constant would describe how quickly and in what ratio the sponge 'gives off' water to the air around it compared to how much water it 'absorbs' from the air.
Practical Implications
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When we evaluate partition constants, we consider how they help us understand the fate and transport of chemicals in systems such as soil-water interactions.
Detailed Explanation
Understanding partitioning is critical to managing chemical pollution and its effects. For instance, if a chemical is spilled on the ground, its concentration in the soil and water can determine how it moves through the environment. The partition constant allows scientists and engineers to predict how quickly and in what amounts chemicals will move between soil and water, which is important for assessing environmental risk.
Examples & Analogies
If someone spills oil on water, the way the oil moves and spreads depends on the partition constant. Understanding this helps responders determine how best to clean up the spill and prevent damage to the environment.
Key Concepts
-
Mass Concentration (Rho): The mass of a substance per unit volume.
-
Mass Fraction (w): The ratio of the mass of focused substance to the mass of solids or other phases.
-
Partition Constant: Represents chemical distribution at equilibrium between two phases, essential for environmental assessments.
Examples & Applications
Example 1: If Rho A1 is the concentration of a chemical in air, and Rho A2 is the same chemical in water, their respective values can be compared to determine the partition constant.
Example 2: In soil studies, rather than calculating volume, researchers will look at mass fractions to simplify the assessment of contaminants.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Rho is for mass, in volume it flows, concentration you gauge, in water or snow.
Stories
Imagine a river where chemicals flow, in air and in water, their balance we know.
Memory Tools
RMP (Rho, Mass fraction, Partition constant) helps you track, how chemicals behave, where they’ll stack.
Acronyms
REM (Rho, Equilibrium, Mass) reminds us what we need to know about concentrations in the field.
Flash Cards
Glossary
- Mass Concentration (Rho)
The mass of a substance per unit volume, expressed in kg/m³.
- Mass Fraction (w)
The ratio of the mass of a substance to the mass of the solid.
- Partition Constant
A ratio describing the equilibrium distribution of a chemical between different phases.
- Aqueous Solubility
The concentration of a substance in water at equilibrium.
- Henry’s Constant
A constant that specifies the ratio of a chemical's concentration in air to its concentration in water.
Reference links
Supplementary resources to enhance your learning experience.
