Nomenclature and Concentration Definitions
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Mass Concentration
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will explore mass concentration, symbolized as Rho. Can anyone tell me what Rho represents in terms of chemical engineering?

Does it represent the mass concentration of a substance?

Exactly! Rho indicates mass per unit volume. For instance, if we talk about Rho A1, what medium do you think is represented?

That should refer to the concentration of A in air!

Correct! And if we switch to water, what would that be?

That would be Rho A2, concentration of A in water.

Well done! To remember, think about 'Rho' as 'Revealing Our Health Opportunities' in environments. Now, let’s recap: Rho is mass per volume, with different subscripts indicating the medium.
Differentiating Between Mass Concentration and Molar Concentration
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s dig deeper. What’s the difference between mass concentration and molar concentration?

Mass concentration is in terms of mass by volume, whereas molar concentration is in moles per volume.

Well spoken! Molar concentration is usually represented by 'C', and in our case, we will focus mainly on Rho. Why do you think focusing on mass concentration is more relevant?

Because in many environmental contexts, we deal with measurable mass, like in pollution studies?

Exactly. It simplifies calculations. Remember the acronym 'MaC'—Mass concentration is crucial for analyzing contaminants and chemical behaviors.
Applications of Partition Constants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss partition constants. They help us understand the distribution of chemicals between different phases, like air and water. Can someone explain how a partition constant is formulated?

Isn’t it a ratio of concentrations in those phases?

Exactly! If we denote it as K, can you tell me how we would represent this for a chemical A distributed between air and water?

It would be K = Rho A1 / Rho A2!

Correct! Remember: 'K is King' when understanding distributions. Always keep the equilibrium concept in mind—when do we add the star in our notation?

When we refer to equilibrium concentrations!

Right! Great job, everyone. Remember, partition constants help elucidate how substances behave in the environment.
Practical Considerations in Soil Analysis
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s conclude with soil concentration analysis. What challenges might we encounter when measuring chemical concentrations in soil?

Soils have variable volumes and compositions, which can make measurements tricky.

Absolutely. That’s why we often use mass fractions instead of volume. If we denote mass fraction as 'w', what might be the equation for concentration in soil?

It would be M A over M 3, right?

Exactly! Always remember that understanding these methodologies is critical for monitoring contamination. Let's remember 'Soil Mean Equations'—SME can help link concepts on measuring in soil.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, the focus is on mass concentration as the primary quantity of interest, introducing various notations for different media. It distinguishes between mass concentration and molar concentration, explains the significance of partition constants, and describes how expressions for chemical concentrations in various phases can differ.
Detailed
In this section, Professor Ravi Krishna introduces key terminologies related to mass concentration and establishes a systematic nomenclature for this course. The notation 'Rho' represents mass concentration, where subscripts refer to different media (e.g., air, water, solids). It emphasizes the importance of understanding these definitions as they underpin calculations and the significance of equilibrium properties, such as aqueous solubility and vapor pressures, indicated with a star symbol to denote equilibrium. Furthermore, the section delves into partition constants, explaining their role in understanding the distribution of chemicals between different phases, such as air and water or water and soil. The importance of mass fractions and the challenge of measuring mass concentration in solids, especially soils, are also discussed, culminating in insights into how contamination dynamics operate over time. Thus, the nomenclature is not only essential for clarity but also fundamental to environmental quality monitoring.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Concentration
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Our main quantity of interest is concentration. We are looking at mass concentration, denoted by the symbol Rho (ρ). This represents the concentration of a substance A in a medium, such as water or air.
Detailed Explanation
Concentration is a key concept in environmental science, particularly in the monitoring of pollutants. Mass concentration, represented by the symbol ρ (Rho), is defined as the mass of a substance per unit volume of the medium in which it is found. For instance, if we have a pollutant in water, its mass concentration would tell us how much of that pollutant is present in a specific volume of water. This is crucial for understanding the potential impact on health and the environment.
Examples & Analogies
Think of a glass of orange juice. If you squeeze in a lot of oranges, the juice becomes thicker. Here, the mass concentration of orange juice increases as more oranges are added—just like how pollutants can increase in water as more waste is dumped into it.
Understanding Mass Concentration Symbols
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
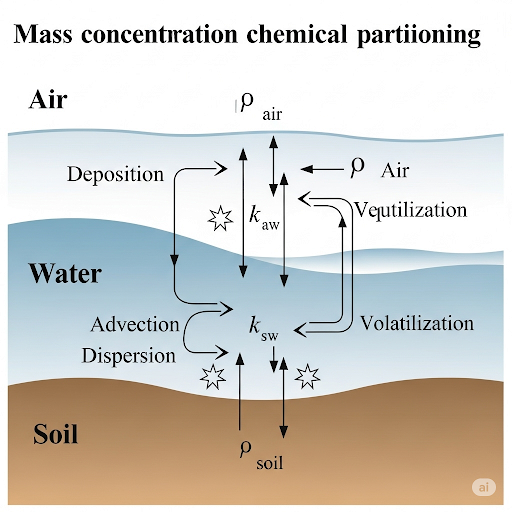
The symbol ρ A represents mass per volume. It indicates concentrations for different media: ρ A1 for air, ρ A2 for water, ρ A3 for solid, and ρ A4 for pure chemical. The density is expressed as mass divided by volume units.
Detailed Explanation
In this context, ρ A represents the mass concentration of substance A in a specified medium. The indices 1, 2, and 3 refer to different media: 1 is air, 2 is water, and 3 is solid material. By specifying the medium, it allows us to differentiate where the substance is located, which is essential for environmental assessments. The mass per volume (ρ) essentially tells us how much of substance A there is in various environments.
Examples & Analogies
Consider measuring sugar in water to create a sweetened drink. The more sugar you add (mass) relative to the volume of water, the sweeter it becomes. Similarly, in an environmental context, if we assess a river and find a high mass concentration of a pollutant, it implies that the river could be very 'polluted.'
Challenges with Soil Concentration Calculations
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For soil, we write ρ A3 as the concentration of A on solid. However, measuring volume of solid is complex due to its porous nature. Therefore, we use mass fraction instead of mass over volume.
Detailed Explanation
Calculating the concentration of a chemical in soil poses unique challenges because of the soil's heterogeneous and porous structure. Direct measurement of the volume of soil can be difficult, particularly in environments like wetlands or beaches, where soil consistency varies. Instead, we often express concentrations in terms of mass percentage (or mass fraction), which simplifies analysis and avoids the difficulties of precise volume measurement.
Examples & Analogies
Imagine trying to weigh a mixture of sand, clay, and rocks in a handful of soil. The volume isn't uniform due to air pockets and varied particle sizes. By focusing on how much mass of a contaminant is in the soil rather than trying to find a precise volume, we simplify the challenge and make the data more usable.
Equilibrium Concentrations and Related Concepts
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Aqueous solubility is expressed as ρ A2 indicated with a star to represent equilibrium concentration. When a chemical A is dissolved in water, we refer to this as an equilibrium property.
Detailed Explanation
In chemistry, equilibrium refers to a state where the concentration of the reactants and products remains constant over time. When we discuss aqueous solubility of a chemical A in water (noted as ρ A2*), we imply that this concentration has reached an equilibrium point—where the rate of dissolution equals the rate of precipitation. This is a critical concept in understanding how pollutants interact with different media.
Examples & Analogies
Think of a bath where you’re adding bath salts. Initially, the salts dissolve quickly, but at a certain point, no more salts can dissolve—this is the equilibrium point. In the environment, a chemical can reach a point where its concentration in water remains stable, despite ongoing interactions with soil or air.
Henry's Constant and Partitioning
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Henry's constant is a crucial ratio that defines the partitioning of chemical A between different phases like air and water.
Detailed Explanation
Henry's constant provides insight into how a chemical behaves between two different phases, such as liquid and gas. Specifically, it defines how much of a chemical will dissolve in water versus how much will remain in the air. This is essential for predicting the distribution of pollutants in the environment, especially for chemicals that tend to evaporate. The equilibrium of concentrations in both phases can help inform regulatory limits and remediation efforts.
Examples & Analogies
Imagine spraying a perfume in a room. Initially, most of the scent is in the air (gas phase), but over time, some may settle on surfaces (liquid phase). The ratio of scent that stays in the air versus what gets absorbed into solid surfaces can be likened to Henry's constant—helping us understand how long smells linger and how they interact with their environment.
Solid-Water Partitioning
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The partitioning of a chemical A between water and solids is given by a specific partition constant (K A32). This indicates how substances interact within soil-water systems.
Detailed Explanation
This partition constant helps describe how a chemical distributes itself between water and solid surfaces, particularly in complex soil environments. The ratio indicates how much of a chemical in water can be found in contact with soil or sediments. Understanding these dynamics is vital for assessing environmental contamination and predicting the movement of pollutants through groundwater or sediments.
Examples & Analogies
Think about oil floating on water. When oil is spilled, some of it drifts and some seeps into the sand at the shore. The partition constant tells us about how this divide happens—helping environmental scientists understand both immediate impacts and long-term effects on ecosystems.
Key Concepts
-
Mass Concentration: The primary focus for determining the amount of substance in a medium.
-
Partition Constants: A fundamental metric for understanding how substances distribute between phases.
-
Equilibrium: Important for context in chemical interactions and behavior in various environments.
Examples & Applications
Rho A1 refers to the concentration of a chemical in the air, while Rho A2 refers to its concentration in water.
The partition constant K between air and water for a chemical A can help predict its movement in the environment.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Rho for mass and 'C' for moles, in air and water, science unfolds.
Stories
Once there was a chemical named A, who loved to travel from air to water, measuring his concentration while he made every wave.
Memory Tools
Rho A1, Rho A2, think of where chemical A’s been, one in air, one in water, keep your studies lean!
Acronyms
Remember 'MCE'—Mass Concentration Equals the measure of pollutants in the air and sea!
Flash Cards
Glossary
- Mass Concentration (Rho)
The mass of a substance per unit volume of solution, represented as Rho (ρ).
- Molar Concentration (C)
The number of moles of solute per unit volume of solution.
- Partition Constant (K)
A ratio that expresses the distribution of a chemical between two phases at equilibrium.
- Mass Fraction (w)
The ratio of the mass of a component to the total mass of the mixture.
- Equilibrium Concentration
The concentration of a substance when it is in equilibrium with another phase, denoted by the symbol with a star ( * ).
Reference links
Supplementary resources to enhance your learning experience.
