Partitioning Between Phases
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Mass Concentration
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome class! Today, we’re starting with mass concentration, represented by ρA. This measures the amount of substance A in a given volume of medium like air or water.

What does the notation ρA1 or ρA2 mean?

Good question! ρA1 indicates the concentration of A in air, whereas ρA2 denotes its concentration in water. The number indicates the phase.

Is there a difference between mass concentration and mole concentration?

Yes, there is. Mass concentration uses mass per volume (like ρA), while mole concentration, represented by C, uses moles per volume.

So why are we focusing on mass concentration in this class?

We’ll focus on mass concentration because it is more practical for environmental monitoring in our context.

Can you give us a summary of what we learned today?

Certainly! We learned that ρA measures mass concentration, with specific notations for different phases, emphasizing its importance for our further studies...
Understanding Partition Constants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's move on to partition constants. Can anyone explain what a partition constant is?

Is it the ratio of concentrations of a substance in different phases?

Exactly! For example, the partition constant K12* relates to the equilibrium between air and water. It explains how a chemical distributes itself between these phases.

What do the stars denote in the notation?

The stars indicate that the values are at equilibrium. This means that the concentration levels have stabilized between the two phases.

How does this relate to environmental monitoring?

It's crucial for predicting how pollutants will behave in the environment and how they might affect ecosystems.

Can we summarize that?

Certainly! Partition constants express the ratios of concentrations at equilibrium and are essential for understanding a chemical's distribution across different phases.
Practical Applications of Partitioning
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s discuss practical applications of what we’ve learned. How do you think partitioning affects groundwater contamination?

If there's a spill, the chemicals will dissolve and move into the soil and groundwater.

That's correct! And which principle here is guiding this movement?

I think it's the partitioning, which determines how much chemical moves from soil into water.

Exactly right! Understanding this partitioning can help us design better remediation strategies.

What happens if the chemical has a higher affinity for solids?

It will likely remain in the soil longer, affecting its bioavailability and potential for impacting water quality.

Can we sum this up clearly?

Yes! Partitioning plays a crucial role in predicting chemical behavior during contamination, informing us on remediation efforts.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Partitioning between phases refers to how chemicals distribute in different environments, such as air, water, or soil. This section explores concepts like mass concentration, partition constants, and the equilibrium between these phases, emphasizing the significance of these concepts in environmental quality monitoring and chemical engineering.
Detailed
Partitioning Between Phases
In this section, we delve into the concept of partitioning of chemicals between different phases, primarily focusing on air, water, and solids. It initiates with a clear nomenclature where mass concentration is denoted with the symbol ρ (rho). The comprehensive understanding includes:
- Mass Concentration Definition: ρA represents the mass of substance A per unit volume in different mediums, with specific indices indicating air, water, and solids.
- Phase Definitions: The indices help identify the phase (i.e., air - 1, water - 2, solid - 3, pure chemical - 4).
- Equilibrium Properties: Concepts like aqueous solubility and vapor pressure are introduced with equilibrium notations (ρ*), indicating their dependence on interactions between phases.
- Henry’s Law: A significant relationship explaining how the concentration of a chemical in one phase relates to its concentration in another through partition constants.
- Partition Constants: The behavior of chemicals between water and solids (i.e., soil) is assessed through specific ratios, depicting their distribution and equilibrium dynamics.
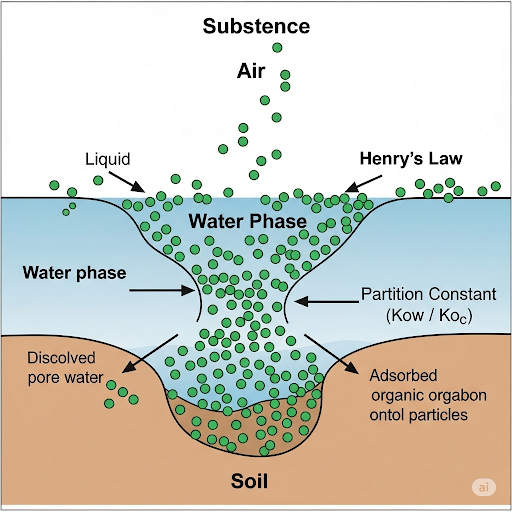
This exploration is crucial for understanding environmental contamination dynamics, where the movement of chemicals impacts ecosystem quality.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Mass Concentration
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Our main quantity of interest is concentration. We are looking at mass concentration denoted by Rho, such as Rho of A in water, air, or some other medium. We use subscripts to indicate the medium, where ‘i’ equals 1 is ‘air’, ‘i’ equals 2 is ‘water’, 3 corresponds to ‘solid’, and 4 corresponds to ‘pure chemical’.
Detailed Explanation
Concentration measures how much of a substance is present in a volume of medium (like air or water). We are specifically looking at mass concentration, which means we express the amount of matter (mass) in terms of volume. Rho (ρ) is the symbol for density, representing this mass per unit volume. For example, Rho A1means the mass of chemical A per volume of air.
Examples & Analogies
Think of this like measuring how much sugar you have in a cup of water. If you dissolve 10 grams of sugar in 1 liter of water, the mass concentration is 10 grams per liter. Just like we label sugar as ‘sugar in water’, we use Rho to label various chemicals in air, water, or solids.
Challenges with Soil Measurements
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When measuring concentration in soil, we might write Rho A3, but directly measuring volume in heterogeneous and porous media like soil can be difficult. Instead, we often use mass fraction instead, denoted as 'w'. This represents the mass of A over the mass of solid.
Detailed Explanation
Soil is not uniform. It contains different sizes and shapes of particles, making it hard to determine its volume accurately. Instead of focusing on the volume of soil, we focus on the fraction of mass that a substance contributes compared to the total mass of the soil. This approach simplifies calculations and makes it easier to understand the behavior of chemicals in soil.
Examples & Analogies
Imagine making a cake with various ingredients. Instead of measuring the exact volume of flour or eggs, you might just care about how much each ingredient contributes to the batter. Similarly, we look at how much chemical is present in relation to the total mass of soil, simplifying the complexity of measuring soil volume.
Defining Equilibrium Concentrations
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Aqueous solubility is measured as Rho A2, indicating the equilibrium concentration of A in water. Vapour pressure, denoted as Rho A1, shows the equilibrium concentration in air. The star signifies that these concentrations are at equilibrium with a particular phase.
Detailed Explanation
Equilibrium concentrations are important because they tell us how much of a substance can exist in different phases at a stable state. When we say Rho A2, we mean that the concentration of chemical A in water has reached a point where it balances with the amount of A in other forms (like vapor or solid). The star () denotes that this value considers the system at equilibrium, ensuring accurate measurements.
Examples & Analogies
Think of a sponge soaking in water. Once the sponge is saturated (at equilibrium), it can no longer absorb more water, no matter how long it stays in it. Similarly, Rho A2* shows when the concentration of a chemical in water can’t increase further without changes in the environment.
Partition Constant and Chemical Behavior
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The partition constant (K) describes the ratio of Rho A1 to Rho A2 under equilibrium conditions. It helps explain how a chemical distributes itself between air and water, allowing us to predict its behavior in different environments.
Detailed Explanation
The partition constant is a way to quantify how much of a substance will be found in one phase compared to another. For instance, if you have a chemical in both air and water, K lets us understand if it prefers to stay in air or dissolve in water. Knowing this helps in predicting how a chemical will act in nature.
Examples & Analogies
Picture a tea bag in a cup of hot water. When you put it in, the tea elements start to dissolve into the water. If you let it steep, the tea favors the water (higher concentration) compared to the remaining tea elements in the bag. This behavior can be described using the partition constant, showing how much tea is in the water compared to the tea bag.
Partitioning Between Water and Solid
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The partitioning of a chemical A between water and solids is crucial. For this, we denote the partition constant as K A32, defined as mass of A in solid (w A3) over mass of A in water (Rho A2). This ratio indicates how much of A is retained in the solid compared to what is in the water phase.
Detailed Explanation
Understanding how chemicals move between water and solids (like soil) is critical for assessing environmental risks. K A32 measures how much of a chemical will stay in the solid phase (like contaminated soil) versus how much will dissolve into the water. This knowledge is vital for managing contamination and pollution in ecosystems.
Examples & Analogies
Imagine a sponge (the solid) immersed in a solution. As the sponge absorbs the solution, some of the solution clings to the sponge while the rest remains in the surrounding water. By understanding how much solution clings to the sponge relative to what is in the water, we can better grasp how contaminants may behave in real-world environments.
Impact of Chemical Properties on Partitioning
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Different soils have varying organic contents and compositions which affect the partitioning of organic chemicals. The organic content of soil plays an important role in how a chemical interacts with the solid phase.
Detailed Explanation
The composition of soil impacts how chemicals partition between soil and water. Organic materials in soil can absorb organic chemicals more effectively, influencing their movement and behavior in the environment. By understanding these properties, we can predict how pollutants will behave when they come into contact with different types of soil.
Examples & Analogies
Consider how different types of sponges (washed and untreated) can absorb liquid differently. An organic-rich sponge might soak up more juice compared to a regular one. Similarly, soils rich in organic matter will likely hold onto organic chemicals better than sandy soils, thus affecting the chemical's behavior in the ground.
Key Concepts
-
Mass Concentration: Measurement of substance concentration in a medium by mass.
-
Partition Constants: Ratios of concentrations of a chemical between different phases crucial for understanding environmental interactions.
-
Equilibrium: The state where concentration balance occurs between two phases.
Examples & Applications
When a chemical spill occurs on land, it can dissolve in water and move into groundwater, determined by its partition constant.
In soil-water systems, the higher organic content in forest soil can attract more organic pollutants compared to sandy beach soil.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In a medium's embrace, concentration finds its place; mass divided by volume, makes our problems race!
Stories
Imagine a chemical at a party, feeling out of place whether in air, water, or soil. It chooses where to mingle based on the partition constants, settling at equilibrium like a well-mannered guest.
Memory Tools
K = C1/C2 helps me see, how chemicals dance from phase to phase with glee!
Acronyms
PEACE
Partition
Equilibrium
Aqueous
Concentration
Environment – the key points we embrace.
Flash Cards
Glossary
- Mass concentration
The mass of a substance per unit volume of a medium.
- Partition constant
The ratio of concentrations of a substance between two different phases at equilibrium.
- Equilibrium
A state where the concentrations of substances in different phases remain constant over time.
- Aqueous solubility
The concentration of a substance dissolved in water, typically at equilibrium.
- Vapor pressure
The pressure exerted by a vapor in equilibrium with its liquid or solid phase.
Reference links
Supplementary resources to enhance your learning experience.
