Equilibrium Properties of Aqueous Systems
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Mass Concentration
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to explore mass concentration, a primary focus in our analysis. The symbol Rho denotes mass concentration. Can anyone tell me what Rho A1 and Rho A2 represent?

Rho A1 is the concentration of a chemical in air, and Rho A2 is in water.

Exactly! And what about Rho A3 and Rho A4?

Rho A3 is for solids, and Rho A4 relates to pure chemicals.

Great job! Remember that Rho corresponds to mass by volume, whereas we typically represent concentration as C, in moles per meter cube. This distinction is crucial in environmental systems.
Understanding Partition Constants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's dive into partition constants. Can anyone explain what a partition constant is?

It’s a ratio that describes how a chemical distributes itself between two phases, like air and water.

That's right! Specifically, we denote it as K, where K is the ratio of Rho A1 to Rho A2 at equilibrium. Why is understanding this ratio important?

It helps us understand how a chemical behaves in different environments, especially when looking at pollution.

Correct! Knowing how chemicals partition can give us insight into their fate and transport in the environment.
Application of Equilibrium Properties
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, how does this knowledge apply in real-world scenarios, such as soil pollution?

Well, if a chemical is dumped on soil, it can dissolve into pore water and later partition to solid particles.

And over time, that chemical can reach groundwater.

Exactly! This movement can lead to contamination if not monitored. What role does organic content in soil play in this context?

Organic content can attract organic pollutants, increasing their accumulation in the soil.

Great insights! As we study these interactions, we see how critical understanding equilibrium properties are for environmental management.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Focusing on the concepts of mass concentration, partition constants, and their applications to chemical interactions in water, air, and soil, this section provides insights into the relevance of equilibrium properties in environmental science.
Detailed
Detailed Summary
This section delves into the equilibrium properties of aqueous systems, particularly examining mass concentration, partition constants, and how these concepts apply to environmental quality and chemical interactions. Concentration is primarily expressed in mass units (denoted as Rho), where Rho A1 represents the concentration of a chemical in air, Rho A2 in water, and Rho A3 in solid media. In this context, mass concentration differs from molar concentration used in other fields.
Key terms include: Rho (density or mass concentration) and K (partition constant), which denotes the equilibrium ratio of concentrations of a chemical between two phases, such as water and air. The significance lies in understanding how chemicals partition between these media, affecting their behavior and fate in the environment.
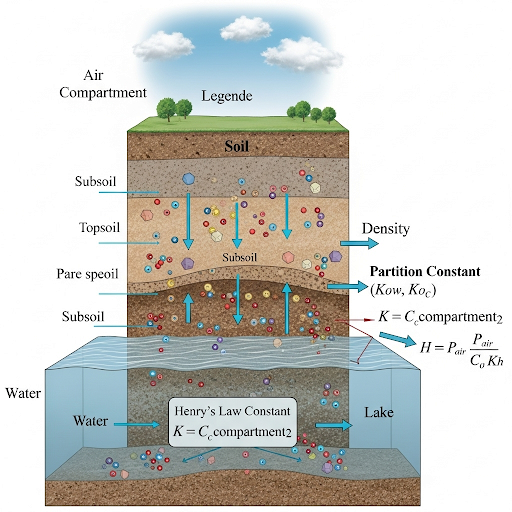
Additionally, the section addresses Henry's constant, which describes the equilibrium concentration of a gas above a liquid, and emphasizes the relevance of understanding these concepts in environmental monitoring. The interactions within soil media and the implications for pollutants traveling through soils to groundwater are also critically discussed. This knowledge is essential for predicting the environmental impact of chemicals and managing contamination.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Concentration Nomenclature
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, we will start today's class with the nomenclature that we are going to follow for this course.
Ah, our main quantity of interest is concentration. And we are looking at mass concentration. So the mass concentration symbol is Rho, so Rho of A and in some medium. So here, I have to put some number here to indicate whether something here to indicate it with air or water.
So I will, so will have “i” here, “i” equals 1 is ‘air’, “i” equals 2 is ‘water’ the symbol 3 corresponds to ‘solid’, 4 corresponds to ‘pure chemical’. This is a general nomenclature.
Detailed Explanation
In this section, we learn about the terminology used to denote concentration in various media such as air, water, and solids. The symbol Rho (ρ) is used to represent mass concentration, which is defined as mass per volume. The lecture highlights that each medium has a specific index: '1' for air, '2' for water, '3' for solids, and '4' for pure chemicals. This nomenclature helps in clarifying the context when discussing concentration in different environments.
Examples & Analogies
Imagine you have a fruit juice that you want to dilute with water. If you say you want a concentration of juice but don’t specify how much juice to how much water, it could be confusing. This is similar to how the index helps clarify what medium we are discussing when we talk about concentration of a substance like a chemical in air, water, or solid form.
Mass Concentration Versus Mass Fraction
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When we say concentration, it is by default in this class is mass concentration. In air when we say Rho A2, this is concentration of A in water.
So, what about soil? So in soil, you can also write Rho A3, just concentration of A on solid. But the problem is some of these, this means we are looking at M of A divided by volume of solid. Lot of times volume of solid is not very easy to obtain.
Detailed Explanation
This chunk clarifies the difference between mass concentration (Rho) and mass fraction. While mass concentration refers to the amount of a substance per unit volume (like Rho A2 for water), obtaining volume measurements for solids can be complicated due to their heterogeneous nature. Instead, it is often more practical to use mass fraction (denoted by 'w' or 'Omega'), which represents the mass of a substance divided by the mass of the solid.
Examples & Analogies
Consider a bag of mixed nuts. It is easier to say that you have 300 grams of almonds compared to trying to measure their volume, especially since they are different shapes and sizes. This illustrates the convenience of using mass fractions in complex mixtures like soil.
Equilibrium Properties: Aqueous Solubility and Vapor Pressure
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In this context, when we will apply this nomenclature to whatever we did in the last class we are to three physical properties. The first one is aqueous solubility. We call it as Rho A2 but we indicate that it as an equilibrium property by putting a star there, ok. So, whenever this star appears anywhere, it is something to do, something in equilibrium with something. It relates to equilibrium; some mention of equilibrium is there.
Detailed Explanation
This section explains how equilibrium properties such as aqueous solubility and vapor pressure are represented. When a star is added next to a symbol (like Rho A2*), it indicates that the concentration is in equilibrium with another phase. Aqueous solubility is the equilibrium concentration of a chemical in water, while vapor pressure relates to the concentration of a chemical in air. These concepts are crucial when studying how substances interact within different environments.
Examples & Analogies
Think of equilibrium like a balancing act. When you have a seesaw, if one side gets heavier, it tips. In the context of chemicals, when a substance dissolves in water, there’s a balance point—where no more substance can dissolve without causing a shift in the system.
Key Concepts
-
Mass Concentration: Defined as mass per unit volume, important in calculating concentrations in aqueous systems.
-
Partition Constant (K): Describes how a chemical partitions between air and water or between solid and aqueous phase.
-
Henry's Constant: Important for understanding how gases behave when in contact with liquids.
Examples & Applications
Example of a chemical being analyzed in soil versus water to understand its partition behavior.
Investigating the effects of pollutants in groundwater and their interaction with soil properties.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
To find Rho in air or water's play, remember K's the key to what chemicals weigh!
Stories
Imagine a traveler named Henry who visits a liquid lake and observes the gases forming above. Understanding his observations fosters awareness of how gases dissolve.
Memory Tools
Remember Rho A1 = Air, Rho A2 = Water with a partition that's K, keeping it in order.
Acronyms
K.E.P. - K (partition constant), E (equilibrium), P (phase behavior).
Flash Cards
Glossary
- Mass Concentration
The quantity of mass of a substance per unit volume of a solution, often represented by Rho.
- Partition Constant
A ratio that describes how a chemical distributes itself between two phases at equilibrium.
- Henry's Constant
A proportionality constant used to describe the amount of dissolved gas in a liquid at equilibrium.
- Aqueous Solubility
The concentration of a solute in water when the solution is saturated at a given temperature.
Reference links
Supplementary resources to enhance your learning experience.
