Analysis Methods – Gas Chromatography
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Chromatography
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're going to explore gas chromatography. Who can tell me what the main components of a chromatography system are?

Isn't it the column and the mobile phase?

Exactly! The column is our stationary phase, while the mobile phase carries the sample through it. Why do you think the mobile phase is critical in the separation process?

I think it allows the components of the sample to be separated based on their different affinities?

Yes! We refer to this difference in affinity as the partition constant, denoted as K. Higher values of K indicate stronger retention in the column. Can someone tell me how this could affect the results we get?

If K is high, the component takes longer to move through the column, right?

Correct! The retention time is crucial for both qualitative and quantitative analyses. Great job!
Factors Affecting Separation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's discuss what factors influence the value of K. Can anyone name one?

Temperature seems important. Does higher temperature decrease K?

Indeed! Increasing temperature generally leads to a lower K value, meaning the compounds exit the column faster. What about the stationary phase? How does that come into play?

Isn’t it difficult to change the stationary phase once it’s set?

Exactly! Changing stationary phases can be costly and impractical. That's why it’s often best to design the system around the specific analysis needs. Now, let’s think about the dynamic possibilities! Why might varying conditions during a single run be useful?

It would help analyze mixtures that have compounds with different affinities more effectively, right?

That's a great point! This optimization is key to increasing throughput and efficiency in analysis.
Types of Columns
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let's differentiate between packed and capillary columns. What do you know about packed columns?

Packed columns are longer but have high pressure drops.

Correct! While they can provide effective separation, the pressure drop can be a major limitation. Now, what about capillary columns?

They are smaller and can be much longer, right? They allow for better efficiency.

Exactly! They minimize pressure drop and maximize separation efficiency due to their design. How does having a longer length contribute to separation?

Longer lengths allow for more adsorption and desorption cycles, improving separation.

Exactly! Great observations!
Detection Methods
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's move on to detection methods. Can anyone name a detector used in gas chromatography?

I know about Flame Ionization Detectors, or FID.

Correct! FID is essential for detecting hydrocarbons. How does it work?

It burns the hydrocarbons and measures the change in resistivity.

Exactly! But what about universal detection? What’s a detector that can analyze multiple types of compounds?

Thermal Conductivity Detector (TCD).

Correct! TCD can measure thermal conductivity differences and is a universal detector. And how about mass spectrometry? Why might that be advantageous?

It provides much more detailed information on the compounds, right?

Exactly! Mass spectrometry gives us not just separation information but identification, which is crucial in analyses.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, the fundamental concepts of gas chromatography are explained, highlighting the importance of the stationary and mobile phases for separation of compounds. It delves into how the partition constant, temperature, and flow rates influence detection. The section also introduces different types of detectors used in gas chromatography.
Detailed
Overview
Gas chromatography (GC) is a widely used analytical technique for separating and analyzing compounds that can vaporize without decomposition. This section discusses the critical components of gas chromatography, including the column, which acts as the stationary phase, and the mobile phase (gas). The separation of components is largely governed by their differing affinities for the stationary and mobile phases, described quantitatively by the partition constant (K).
Key Components and Separation Mechanism
- Column: This is the heart of the chromatographic system, where the separation occurs. The stationary phase is fixed within the column, and the mobile phase, often an inert gas, carries the sample through the column.
- Partition Constant (K): The separation efficiency relies on K; a higher K indicates stronger retention in the column, while a lower K leads to quicker elution.
- Factors Affecting Separation: Temperature and the choice of stationary phase and mobile phase influence K. Higher temperatures typically lower K, causing faster elution.
- Dynamic Operation: In practical applications, different conditions can be applied dynamically during a single analysis run for efficient separation of complex mixtures.
Types of Columns
- Packed Columns: Generally longer (1-2 meters) but suffer from high pressure drop, limiting their effectiveness for separations.
- Capillary Columns: Smaller diameter with higher lengths (up to 60 meters), offering better separation efficiency because of reduced pressure drop, allowing more surface area for adsorption.
Column Temperature Control
In gas chromatography, temperature plays a crucial role in optimization. Temperature programming allows for flexibly adjusting conditions throughout an analysis.
Detection Methods
Different detectors are utilized in gas chromatography, each with its advantages and limitations:
- Flame Ionization Detector (FID): Best for hydrocarbons, measuring changes in resistance caused by combustion of eluted compounds.
- Thermal Conductivity Detector (TCD): A non-specific universal detector that measures variations in thermal conductivity of the gas exiting the column.
- Electron Capture Detector (ECD): Highly sensitive to halogens, useful for environmental analysis.
- Mass Spectrometry (MS): Provides detailed compositional information beyond separation, allowing for compound identification through mass spectra.
Understanding these principles enables the efficient application of gas chromatography in various analytical contexts, ensuring accurate and reliable results.
Youtube Videos






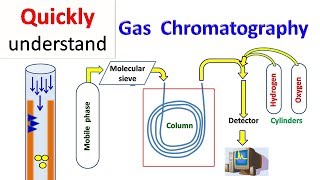



Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Gas Chromatography
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The main part of the chromatography system is the column which is also called as a stationary phase and there is also what is called as a mobile phase. So, the purpose of the mobile phase here is, you introduce the sample, a mixture which is usually a pulse or finite volume just before the column and then you have the separated components coming out of the column which are then detected. The main purpose of the column is the separation.
Detailed Explanation
In gas chromatography, there are two main components: the stationary phase (the column) and the mobile phase (the gas). The mobile phase carries the sample mixture to the column. The column is where the separation of different components of the mixture occurs. When the sample enters the column, each component interacts differently with the stationary phase, leading to their separation based on their unique characteristics.
Examples & Analogies
Imagine a school bus (the mobile phase) dropping off students (the mixture) at different houses along a street (the stationary phase). Each house represents a different attraction for the students; some students prefer some houses over others, making them get off at different times. Just like the students get separated based on their preferences, the components in the mixture are separated as they pass through the column.
Separation Mechanism
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The separation occurs mainly because it takes advantage of different affinities of the analyte between the stationary phase and mobile phase. So, in other words, we are talking about some partition constant between the stationary phase and the mobile phase. So, the extent of the separation depends on the type of the affinity.
Detailed Explanation
The principle behind the separation in gas chromatography relies on the varying affinities of different components (analytes) for the stationary phase and mobile phase. This is quantified by a value known as the partition constant (K). If a compound has a high K value, it means it has a greater affinity for the stationary phase and will spend more time in the column, thereby separating from other compounds that have lower K values.
Examples & Analogies
Think of a dance competition where dancers (analytes) are judged by different judges (the stationary phase) based on their skills (affinities). The dancers who perform well will get higher scores (higher K values) and spend more time being judged, leading to a final ranking that separates them based on their performances (separation of components).
Factors Affecting Separation
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
To control separation you can manipulate 2 factors: one is the retention and the other is partitioning constant.
Detailed Explanation
Two primary factors influence how well separation occurs in gas chromatography: retention time and the partitioning constant. Adjusting the temperature can affect these factors. For instance, higher temperatures generally lead to lower retention times because analytes will pass through the column more quickly since they interact less with the stationary phase. Meanwhile, changing the stationary phase itself can also affect the separation but is more complex and cost-prohibitive.
Examples & Analogies
Imagine adjusting the temperature in a room to affect people's comfort while they are waiting for an event to start. If it's too hot, people may leave (lower retention), but if it's just right (optimal conditions), they will stay longer (higher retention), allowing time for better interactions with each other (better separation).
Dynamic Conditions and Optimization
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
We want to do this dynamically in a given sample. So normally when you inject a sample, it starts the beginning of what is called as a sample run... the goal typically is to optimize the analysis because an analysis takes time and analysis costs money.
Detailed Explanation
Optimizing gas chromatography involves adjusting conditions dynamically to achieve the best separation of various compounds in a single sample run. By carefully manipulating conditions, such as the temperature or the flow rate of the carrier gas, one can enhance the separation efficiency and reduce analysis time, ultimately minimizing costs and maximizing throughput.
Examples & Analogies
Consider a chef preparing multiple dishes in a busy restaurant. By adjusting the cooking times and temperatures for different dishes, the chef ensures that everything is ready to serve at the same time. This strategic planning saves time and focuses on delivering a high-quality meal efficiently, much like optimizing the analysis in chromatography.
Types of Detectors in Gas Chromatography
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In this context, chromatography analysis, the mobile phase is a gas typically nitrogen, helium or argon or inert gases... we have a few commonly used detectors. The first one is called as FID; the flame ionization detector.
Detailed Explanation
Different detectors serve various purposes in gas chromatography. The most common detectors include the Flame Ionization Detector (FID), which is highly suitable for analyzing hydrocarbons since it detects the presence of ions created by combusting the compounds. Other detectors measure thermal conductivity or selectively detect specific types of compounds, helping in a diverse range of applications from environmental monitoring to industrial quality control.
Examples & Analogies
Think of detectors in chromatography like different types of security cameras in a mall. The FID is like a camera that focuses on detecting people with specific clothing styles (hydrocarbons), while another might be designed to pick up heat signatures (thermal conductivity), capturing all warm bodies regardless of clothing.
Calibration and Quantification
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In gas chromatography for calibration I have the chromatogram... using the area of the 4 points, I can draw a calibration chart.
Detailed Explanation
Calibration in gas chromatography involves creating a reference chart where known amounts of a substance produce measurable signals (areas under the peaks in a chromatogram). By injecting varying concentrations of these substances, a calibration curve is established, which allows for the quantification of unknown samples based on their detector responses.
Examples & Analogies
Imagine a teacher grading a student’s essay based on a rubric. The teacher develops a standard measurement (the rubric) for different aspects of writing quality. By using this rubric to assess multiple essays, the teacher can establish a pattern for grading, which helps to fairly evaluate future essays based on established criteria, similar to calibrating a chromatogram.
Key Concepts
-
Separation Mechanism: Gas chromatography separates compounds based on the partitioning of analytes between stationary and mobile phases.
-
Partition Constant (K): Determines how long compounds retain in the column based on their affinities for stationary and mobile phases.
-
Detection Methods: Various detectors used in GC (FID, TCD, ECD, MS) provide sensitivity and specificity based on the compounds analyzed.
Examples & Applications
An example of gas chromatography in environmental testing could be analyzing the concentration of volatile organic compounds (VOCs) in air samples.
In petroleum refining, gas chromatography is used to separate and quantify hydrocarbon mixtures.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In chromatography, a gas takes flight, separating compounds with all its might.
Stories
Imagine two friends running a race, one on a track that retains them longer. The faster they run, the less they compare; their bonds of affinity lead to different fair.
Memory Tools
Remember K as 'Keep' - higher values mean compounds keep coming out slowly, while lower K means fast!
Acronyms
GAS
Gaseous mobile phase
Affinity differences
Separation achieved.
Flash Cards
Glossary
- Chromatography
A method for separating components of a mixture based on differences in their movement through a stationary phase.
- Mobile Phase
The phase in chromatography that moves compounds through the stationary phase; in gas chromatography, it is typically an inert gas.
- Stationary Phase
The fixed phase in chromatography that interacts with the analytes; it does not move.
- Partition Constant (K)
The ratio that describes the affinity of a compound for the stationary phase versus the mobile phase.
- Retention Time
The time it takes for a particular analyte to pass through the chromatography system and be detected.
- Flame Ionization Detector (FID)
A detector that measures changes in electrical conductivity caused by burning hydrocarbons.
- Thermal Conductivity Detector (TCD)
A universal detector that measures variations in thermal conductivity of the gas exiting the column.
- Capillary Column
A type of chromatography column with a small diameter, allowing for greater efficiency and separation.
- Mass Spectrometry (MS)
An analytical technique used to identify the composition of a sample by measuring its mass-to-charge ratio.
- Capillary Columns
Thin, long columns used in chromatography that enable efficient separation due to decreased pressure drop.
Reference links
Supplementary resources to enhance your learning experience.
