Gas Chromatography Detectors
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Detectors
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to delve into the types of detectors used in gas chromatography. Can anyone tell me why we need these detectors after the chromatography column?

To separate the different components of a mixture?

Great start! Detectors are crucial as they help us identify and quantify the separated components. Remember, each type of detector has its strengths and weaknesses. Let’s explore the Flame Ionization Detector, or FID.

What does the FID actually measure?

Excellent question! The FID measures the electrical changes resulting from burning hydrocarbons in a flame. It detects based on how much hydrocarbon is present.

So it can only detect hydrocarbons then?

Exactly, it is a non-selective detector for hydrocarbons, meaning it doesn't give specific identification but measures the hydrocarbon concentration.

What if we need to detect something other than hydrocarbons?

This is where we can use different detectors, like the Thermal Conductivity Detector. Let’s explore that next.

To summarize today, we discussed the role of detectors in gas chromatography, focusing on FID and its selective detection of hydrocarbons.
Thermal Conductivity Detector (TCD)
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s talk about the Thermal Conductivity Detector. Who can tell me its principle of operation?

Does it measure temperature changes?

You're on the right track! TCD measures the change in thermal conductivity between the carrier gas and the analytes in the sample. It’s universal, meaning it can detect various compounds.

How sensitive is it compared to the FID?

Good point, TCD is less sensitive than FID. While it can detect various gases, it may not pick up trace amounts as effectively.

What would be a situation where we might use TCD instead of FID?

If we are interested in analyzing air samples that may contain hydrogen or oxygen, TCD would be more suitable.

To recap, TCD offers a universal solution for thermal conductivity measurement, but with lower sensitivity compared to FID.
Electron Capture Detector (ECD)
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, we have the Electron Capture Detector, or ECD. Any idea why it's particularly useful?

Is it similar to FID?

Not quite! ECD is very sensitive to halogens, such as chlorine and bromine. This makes it particularly valuable in environmental applications.

So this would work great for detecting chlorinated compounds?

Absolutely! ECD is excellent for detecting low concentrations of these types of compounds.

But is it non-selective like FID?

Yes, it is non-selective for most analytes but very specific for halogens. Let’s summarize that.

In summary, the ECD is vital for detecting halogenated compounds and can operate at very low concentrations, making it crucial for environmental analysis.
Mass Spectrometer (MS)
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let’s discuss Mass Spectrometry. How does this device stand out compared to others in gas chromatography?

Does it give more detailed information on the compounds?

You bet! MS provides not just the presence of a molecule but also structural data through fragmentation patterns.

Does that mean it can identify compounds better?

Exactly! By generating a mass spectrum, we can differentiate compounds that might otherwise present similar retention times.

So, is MS the most advanced option for analysis?

In many ways, yes! It integrates both qualitative and quantitative capabilities but requires specialized knowledge and calibration.

To summarize, mass spectrometry enriches gas chromatography by providing in-depth identification and quantification through mass-to-charge analyses.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
It details the different types of detectors employed in gas chromatography, including Flame Ionization Detectors (FID), Thermal Conductivity Detectors (TCD), Electron Capture Detectors (ECD), and Mass Spectrometers (MS). Each detector is explored regarding its working principle, detection capabilities, and suitability for analyzing different compounds, particularly hydrocarbons and halogens.
Detailed
In gas chromatography, detectors play a critical role in identifying and quantifying the components of a sample after separation occurs within the chromatography column. Among the key detectors discussed are:
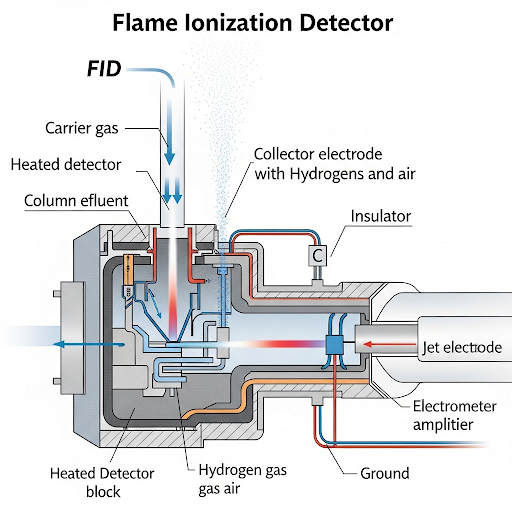
- Flame Ionization Detector (FID) - Primarily used for hydrocarbon analysis, it operates by burning the sample in a flame and measuring the electrical changes that result from ionized particles. It is a non-selective detector that provides a response based on the amount of hydrocarbon present.
- Thermal Conductivity Detector (TCD) - A universal detector that measures the difference in thermal conductivity between the carrier gas and the substances present in the sample. Although it can detect a variety of gases, its sensitivity is lower compared to FID.
- Electron Capture Detector (ECD) - Highly sensitive to compounds containing halogens, making it suitable for the detection of chlorinated compounds, often used for environmental analysis.
-
Mass Spectrometer (MS) - An advanced detector that provides structural information by fragmenting molecules and analyzing their mass-to-charge ratios, offering deeper qualitative and quantitative data compared to other detectors.
The importance of understanding the selection of the appropriate detector is emphasized, as it directly impacts the accuracy and reliability of the analytical results in various applications.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Flame Ionization Detector (FID)
Chapter 1 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The first one is called as FID; the flame ionization detector. It is mainly used for hydrocarbon analysis. What the flame ionization detector does in general is based on some kind of electrical measurement. The signals are all electrical measurement so in this case, there are 2 contact points. In between this whatever sample is coming out will burn.
Detailed Explanation
The Flame Ionization Detector (FID) is a common detector used in gas chromatography specifically for detecting hydrocarbons. It operates by burning the sample as it comes out of the GC column. When hydrocarbons burn in the flame, they ionize and generate charged particles. There are two electrodes, or contact points, placed in the flame that measure the resulting electrical current. The amount of current produced corresponds to the amount of hydrocarbons present: more hydrocarbons mean a larger signal. This method is useful because it provides real-time responses as samples elute from the column, creating peaks in a chromatogram for each compound.
Examples & Analogies
You can think of the FID like a campfire where different materials are thrown in. If you throw in dry leaves (hydrocarbons), they will ignite and produce a big flame, just like hydrocarbons increase the signal in the FID. In contrast, if you throw in a water-soaked log (non-hydrocarbon), it will smolder and not produce much of a flame, akin to the detector not responding to compounds that do not burn.
Understanding FID Signal Response
Chapter 2 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When it burns it changes the resistivity between these 2 electrical points. So, it is measured as a signal that comes up and if you have more hydrocarbons more burning happens and therefore, the signal goes up and comes down.
Detailed Explanation
The key to understanding how the FID works is its response to the combustion of hydrocarbons. As the hydrocarbons burn, they form ions, resulting in a change in electrical conductivity between the two contact points. This change generates a signal, which is recorded over time. In a typical chromatogram, the initial baseline represents the signal from the carrier gas. When a hydrocarbon sample starts to elute from the column, the flame burns hotter due to combustion, resulting in an increase in the recorded signal (peak). As the sample concentration decreases, the signal drops back to the baseline level.
Examples & Analogies
Imagine a concert where the lead singer (the hydrocarbon) takes the stage and sings (burns in the flame), causing the audience (the FID signal) to cheer more loudly. The louder the cheers (higher the signal), the more engaged the audience is. As the singer finishes and walks off stage, the audience's cheer levels decrease back to normal (the baseline).
Qualitative and Quantitative Analysis
Chapter 3 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
One is called as a qualitative analysis. Qualitative analysis answers the question what is the analyte and quantitative you are asking the question how much.
Detailed Explanation
In gas chromatography, two main types of analysis can be performed: qualitative and quantitative analyses. Qualitative analysis helps identify the substance being analyzed, answering the question of 'what is present?' For instance, if two compounds come out at specific times, a chemist can determine what they are by comparing them to known standards. On the other hand, quantitative analysis determines how much of that substance is present, answering the question 'how much is there?'. This is typically done using calibration curves that correlate response signals (like peak area) to the concentration of known standards.
Examples & Analogies
Think of a detective investigating a case. Qualitative analysis is like identifying if a specific person was present at the crime scene by examining fingerprints or DNA evidence (who was there?). Quantitative analysis is like determining how many people were at the scene by counting known witnesses (how many were there?).
Limitations of FID
Chapter 4 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
FID does not care what the compound is as long as there is something burning. It is what we call as a non-selective detector.
Detailed Explanation
One significant limitation of the Flame Ionization Detector (FID) is that it is a non-selective detector. This means it does not differentiate between various types of hydrocarbons; it merely detects any compound that can burn and produce ions. While this sensitivity can be beneficial for hydrocarbon analysis, it could be problematic if a sample contains multiple compounds. If two different hydrocarbons elute from the column at the same time, the FID will produce a single peak, complicating the identification process.
Examples & Analogies
This is like a security guard at an entrance who only checks for people entering but does not care if they are carrying bags, food, or any specific items. They only ensure that someone enters. Similarly, the FID sees a compound burning but does not care about its identity or specific makeup.
Thermal Conductivity Detector (TCD)
Chapter 5 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The second type of detector that you would have seen is what is called as thermal conductivity detector or a TCD. This one is also non-selective which means that it does not give you any specific information about the compound analyte.
Detailed Explanation
The Thermal Conductivity Detector (TCD) is another type of non-selective detector used in gas chromatography. Unlike the FID, which specifically detects burning hydrocarbons, the TCD measures the thermal conductivity of the gas sample exiting the column. It operates by comparing the thermal conductivity of the sample gas to a reference, typically the carrier gas. Any difference in thermal conductivity produces a signal, which can indicate the presence of various compounds, including non-hydrocarbons. However, it is less sensitive than the FID, making it better suited for certain types of analyses.
Examples & Analogies
Imagine measuring the temperature of two liquids - one warm and one cold - using a sensitive thermometer. If the thermostat detects a slight temperature difference, it can suggest the presence of a warm liquid. Similarly, the TCD detects differences in thermal conductivity between the carrier gas and sample gas to signal the presence of different compounds.
Electron Capture Detector (ECD)
Chapter 6 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The third kind of detector is called an electron capture detector or an ECD this is very specific to halogens and has very high sensitivity to halogens, specially chlorinated compounds.
Detailed Explanation
The Electron Capture Detector (ECD) is highly sensitive and specifically designed to detect halogenated compounds, particularly those that are chlorinated. It works by capturing electrons from the ionization of the analytes, which subsequently causes a change in conductivity. This capability makes ECD exceptionally useful in environmental monitoring where halogenated compounds are prevalent. It can detect even trace amounts of these compounds, providing critical information for assessment and analysis.
Examples & Analogies
Consider a very keen person with a heightened sense of smell who can detect even the faintest scent of a specific perfume from a crowded room. Similarly, the ECD can sense the smallest amounts of halogenated compounds in a mixture, flagging them for analysis just like that person identifies the perfume.
Mass Selective Detector (MS)
Chapter 7 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, there is a fourth kind of detector that takes the disadvantages of all of this and it can give you a little more information called as a mass spectrometer or a mass selective detector.
Detailed Explanation
The Mass Selective Detector (MS), or mass spectrometer, is an advanced detector that enhances the analysis capabilities of gas chromatography. It does not only provide data regarding the quantity of a substance, as with traditional detectors, but it also offers detailed information about the molecular weight and structure of the compounds. Every molecule that passes through the mass spectrometer is ionized and fragmented, producing a mass spectrum which serves as a unique fingerprint for each compound, allowing for accurate identification.
Examples & Analogies
Think of a restaurant menu where each dish represents a different compound. A standard detector could tell you how much food is ordered but not what the dishes are. A mass spectrometer, on the other hand, enables you to both see the number of dishes and understand their individual ingredients, allowing specific identification of what has been ordered.
Key Concepts
-
Detector Functionality: Detectors play a crucial role in identifying and quantifying separated components in chromatography.
-
FID: Measures ionization in hydrocarbon burning; non-selective for hydrocarbons.
-
TCD: Measures thermal conductivity differences; universal but less sensitive.
-
ECD: Highly sensitive to halogens; used for environmental analysis.
-
MS: Offers detailed structural information through mass spectra.
Examples & Applications
Example of FID: Use in measuring the concentration of different hydrocarbons in a petrochemical sample.
Example of ECD: Detection of toxic chlorinated solvents in environmental water samples.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
FID detects the flame's glow, hydrocarbons in the flow.
Stories
Imagine a busy lab where experts use different detectors; FID watches the flame for hydrocarbons, TCD measures the temperature dance, while ECD searches for halogens, and MS unveils secrets through masses.
Memory Tools
To remember the detectors: 'Fabulous FID Fights Hydrocarbons, TCD Treads on Thermal, Elegant ECD Enforces on Halogens, Mighty MS Measures Masses!'
Acronyms
F, T, E, M - for FID, TCD, ECD, and MS.
Flash Cards
Glossary
- Flame Ionization Detector (FID)
A non-selective detector used in gas chromatography that measures the ionization of substances as they burn in a flame.
- Thermal Conductivity Detector (TCD)
A universal detector that measures the difference in thermal conductivity between a carrier gas and the analytes present.
- Electron Capture Detector (ECD)
A detector that is highly sensitive to halogenated compounds, useful in environmental analysis.
- Mass Spectrometer (MS)
An advanced detector that provides molecular mass information and structural data by analyzing fragments of ionized compounds.
- Retention Time
The time taken for a particular analyte to pass through the chromatography system and reach the detector.
Reference links
Supplementary resources to enhance your learning experience.
