Monitoring and Analysis
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Gas Chromatography
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we will discuss gas chromatography. Can anyone explain what chromatography is and why it's important?

Isn't chromatography a technique to separate different components of a mixture?

Exactly! Chromatography separates mixtures into individual components based on their interactions with a stationary phase and a mobile phase.

What are the stationary and mobile phases in gas chromatography?

The stationary phase is typically a column with a specific packing, while the mobile phase is usually an inert gas. This setup allows the separation based on component affinities.

What is partition constant?

Great question! The partition constant, K, indicates how a compound interacts with the stationary phase compared to the mobile phase.

So, a higher K means a stronger interaction and longer retention time?

Absolutely! Remember that the goal is to optimize these factors to achieve effective separations. Anything specific you would like to clarify?

Let's recap: Gas chromatography relies on the interaction between stationary and mobile phases to separate compounds effectively.
Factors Affecting Separation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's delve deeper into the factors affecting separation. What can you tell me about how temperature influences chromatography?

I think raising the temperature lowers the retention time, right?

Correct! Higher temperatures typically decrease K, causing faster elution of compounds.

What about the stationary phase? How do we choose it?

Choosing a stationary phase depends on the types of analytes you need to separate and might be limited by cost and availability.

And flow rate? How does that play a role?

Flow rate impacts both separation and analysis time; too high a flow can compromise separation efficiency due to insufficient mass transfer.

To summarize, temperature, stationary phase, and flow rate are crucial for optimizing gas chromatography. Each factor plays an essential role in separation efficiency.
Detection Methods
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's discuss the various detectors used in gas chromatography. What types can you recall?

I remember FID and TCD.

Great! FID, or Flame Ionization Detector, is widely used for hydrocarbon analysis due to its sensitivity.

What about TCD? How does it work?

The Thermal Conductivity Detector measures differences in thermal conductivity from the carrier gas. This detector is universal but not as sensitive as FID.

Are there other detectors that might be used?

Yes! Electronic Capture Detectors are highly sensitive to halogens, and Mass Spectrometers provide detailed spectral data for compound identification.

In conclusion, understanding the functionalities of different detectors allows us to choose the right one based on the analysis needs.
Retention Time and Calibration
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Lastly, let’s discuss retention time and calibration. Can anyone explain why retention time matters?

Retention time helps in identifying the compounds, right?

Exactly! Consistent retention times under the same conditions indicate the presence of specific compounds.

How do we ensure our analysis is accurate using calibration?

Calibration involves injecting standard amounts of known analytes and measuring their response, thus allowing us to create a calibration curve.

What happens if the standards don’t match the unknown samples?

If retention times overlap, it may create ambiguity, and standards help to confirm identities. Always run standards alongside unknowns to improve accuracy.

To summarize, retention time is vital for identification, and calibration is critical for quantification, ensuring precision in results.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The text delves into the mechanisms and methods of gas chromatography, emphasizing the roles of the stationary phase, mobile phase, and factors such as temperature, flow rate, and partition constant in the separation of chemical components. It discusses the types of columns used and various detectors, as well as the importance of retention time and calibration in qualitative and quantitative analysis.
Detailed
Monitoring and Analysis: Gas Chromatography
Overview
Gas chromatography (GC) is a powerful analytical method for separating and analyzing compounds that can vaporize. In this section, we explore the main components and mechanisms of gas chromatography, focusing on how separation occurs in the chromatography column.
Key Components of Gas Chromatography
- Stationary Phase: The column within which the separation happens, typically containing a packing material that interacts with components in the sample mixture.
- Mobile Phase: Usually an inert gas (like nitrogen or helium), it transports the sample through the column, allowing for the separation based on the affinities of components with the stationary phase.
Separation Mechanism
The ability to separate compounds relies on the differing affinities (partition constants, K) of each analyte between the stationary and mobile phases. Higher values of K indicate stronger interactions with the stationary phase, leading to longer retention times.
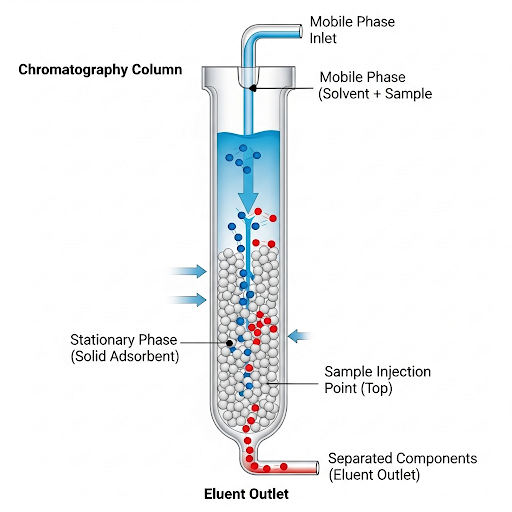
Factors Influencing Separation:
- Partition Constant (K): Indicates the retention characteristics.
- Temperature: Increasing the temperature typically reduces K, allowing compounds to elute faster.
- Stationary Phase: Selection is crucial; however, changing it is often impractical due to cost.
- Dynamic Conditions: The goal is to optimize conditions throughout the run to achieve effective separation while minimizing analysis time and cost.
- Velocity/Flow Rate: Balancing flow rate is necessary to allow sufficient mass transfer while ensuring quick results.
Column Types
- Packed Columns: Typically longer but have higher pressure drops, limiting the effectiveness of separation due to high pressures.
- Capillary Columns: Smaller diameter and longer lengths allow for better resolution and efficiency, although care must be taken with flow rates.
Detection Methods
- Flame Ionization Detector (FID): Commonly used for hydrocarbons; detects based on ionization in a flame.
- Thermal Conductivity Detector (TCD): Universal but less sensitive, detecting compounds based on thermal conductivity differences.
- Electron Capture Detector (ECD): Highly sensitive to halogenated compounds.
- Mass Spectrometer (MS): Provides detailed mass spectra for compound identification.
Calibration and Retention Time
Using known standards for calibration helps in quantifying unknown samples by determining response areas of peaks in chromatograms. Retention times are crucial for identifying analytes, but similar times for different compounds may lead to ambiguity.
In conclusion, mastery of gas chromatography requires an understanding of the underlying principles of separation and detection methods, allowing for effective analysis of complex mixtures.
Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Chromatography
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Talking about chromatography in the previous class. So here the main part of the chromatography system is the column which is also called as a stationary phase and there is also what is called as a mobile phase. So, the purpose of the mobile phase here is, you introduce the sample, a mixture which is usually a pulse or finite volume just before the column and then you have the separated components coming out of the column which are then detected.
Detailed Explanation
Chromatography is a technique used to separate components in a mixture. It involves two key parts: the stationary phase (the column) and the mobile phase (the solvent or gas that carries the sample). When a mixture is introduced into the system, the mobile phase carries it through the column where the different components are separated based on their interactions with the stationary phase. This process allows us to identify and analyze the individual components of the mixture.
Examples & Analogies
Imagine making a fruit salad. You have various fruits (the mixture) that you want to separate. You could use a sieve (the stationary phase) where some fruits will pass through easily (they interact less with the sieve), while others may take more time to pass through because they stick a bit (they interact more). Once separated, you can easily identify each fruit on the plate.
Mechanism of Separation
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So the separation occurs mainly because it takes advantage of different affinities of the analyte between the stationary phase and mobile phase. So, in other words, we are talking about some partition constant between the stationary phase and the mobile phase.
Detailed Explanation
The degree of separation in chromatography relies on the affinity each component of the mixture has with the stationary and mobile phases. This affinity is quantified by a value known as the partition constant (K). Components with a higher K value are retained longer in the stationary phase, leading to longer retention times in the column, while those with lower K values are washed out more quickly by the mobile phase.
Examples & Analogies
Think of a classroom where students (the analytes) prefer different types of seating (the stationary phase). Some students feel more comfortable sitting in the front (they have a high affinity for that spot) and take longer to get up and leave, while others enjoy sitting in the back and leave quickly (they have a low affinity). How long each student stays depends on their comfort and preference, just like how mixtures separate based on their affinity during chromatography.
Factors Affecting Separation
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, just to give you an example, higher the value of K the higher retention in the column, lower K means low retention in the column. It means if you are able to somehow manipulate the retention time in the column, then you can possibly separate some of the components that are in the mixture based on the way in which we manipulate them. So, to control separation you can manipulate 2 factors: one is the retention and the other is partitioning constant.
Detailed Explanation
The partition constant (K) plays a crucial role in how long a component will be retained in the chromatographic column. By manipulating factors such as temperature or the type of stationary phase, one can effectively influence K, thus controlling how components separate. A high retention means the component interacts strongly with the stationary phase, while low retention means it passes through more quickly, influenced by the conditions of the analysis.
Examples & Analogies
Consider a bakery where different types of dough (the analytes) are being baked in ovens (the stationary phase). If you keep the temperature high, some dough types might cook faster and come out quickly (low K), while others will require more time and patience (high K). By adjusting the temperature, you can decide which doughs get done first, similar to controlling separation in chromatography.
Dynamic Manipulation of Separation Conditions
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, we want to do this dynamically. We want the opportunity to dothis dynamically in a given sample. So, in a given sample means suppose you have you have 100 analytes in a mixture, you want to keep adjusting parameters to optimize the separation in real-time.
Detailed Explanation
In chromatography, especially during complex mixtures, it’s beneficial to adjust conditions dynamically to achieve optimal separation of multiple analytes. If analyzing a sample with many components, like a complex extraction from the environment, one would vary parameters such as temperature and flow rate to enhance separation and minimize analysis time. The goal is to analyze as many components as possible efficiently within a single run.
Examples & Analogies
Imagine you are a chef preparing a multi-course meal (the sample) for a banquet. Each dish requires different cooking times and temperatures. To ensure everything is ready at the same time, you might dynamically adjust your oven settings and use multiple pots on the stove. This way, you optimize the cooking process to have all the dishes perfect and ready to serve together, just as a chromatography method should optimize its conditions for different analytes.
Key Concepts
-
Gas Chromatography: A technique for analyte separation using a mobile gas phase.
-
Stationary Phase: The fixed phase in the column that interacts with sample components.
-
Mobile Phase: The gas that carries the sample through the column.
-
Partition Constant (K): Represents the distribution of the analyte between stationary and mobile phases.
-
Retention Time: The duration for which a compound is retained in the column before it exits.
Examples & Applications
When analyzing a sample of air for pollutants, GC could be used to separate various hydrocarbons and identify their concentrations.
In a lab setting, GC is utilized to analyze the purity of a chemical compound by separating impurities based on their boiling points.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In the gas column, components dance, / The mobile phase leads, giving them a chance.
Stories
Imagine a race where compounds are runners and the stationary phase is the track. They dash through the mobile phase, with each runner’s speed depending on their affinity for the track.
Memory Tools
Remember 'Silly Mice Play Very Carefully' for Stationary phase, Mobile phase, Partition constant, Velocity, and Calibration.
Acronyms
K for Knowledge of interaction between stationary and mobile phases.
Flash Cards
Glossary
- Gas Chromatography
A method for separating and analyzing compounds that can be vaporized without decomposition.
- Stationary Phase
The material inside the column that interacts with sample components to affect separation.
- Mobile Phase
The gaseous medium that carries the sample through the chromatographic column.
- Partition Constant (K)
A ratio that indicates the affinity between the analyte and stationary vs. mobile phase.
- Retention Time
The time taken by a compound to travel through the column to the detector.
- Flame Ionization Detector (FID)
A detector used primarily for hydrocarbons that measures the ions produced by burning the sample.
- Thermal Conductivity Detector (TCD)
A universal detector that measures the thermal conductivity of effluent gases.
- Electron Capture Detector (ECD)
A highly sensitive detector used for halogenated compounds.
- Mass Spectrometer (MS)
An analytical tool that measures the mass-to-charge ratio of ions for compound identification.
Reference links
Supplementary resources to enhance your learning experience.
