Factors Affecting Separation
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Chromatography
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome, class! Today we will discuss chromatography, particularly focusing on the factors affecting separation. Can anyone tell me what chromatography is?

Is it a technique used to separate mixtures?

Exactly! Chromatography separates components based on their affinities for two phases: the stationary phase, usually a column, and the mobile phase. Why do you think these affinities are important?

Because they determine how long each component stays in the column?

Right! This leads us to the partition constant, K. A higher K value means greater retention. What happens with a lower K value?

The component will come out of the column faster!

Good job! Remember this with the mnemonic 'Higher K, Slow Down; Lower K, Speed Up.' Now, let's explore how temperature affects K.
Temperature and Partition Constant
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

When we increase temperature, we often see a decrease in K. Can someone explain why this is significant?

It means at higher temperatures, components will separate more quickly!

Precisely! High temperature can lead to faster separations. But be mindful; too high could affect the separation quality. This is where it’s crucial to find the right balance. What’s another way to influence separation?

Changing the stationary phase, but that can be complicated and expensive.

Yes! While changing stationary phases could optimize our analysis, it isn't practical. So we often stick to generic columns. Let’s now see how we can achieve optimal conditions dynamically.
Dynamic Adjustments in Chromatography
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

In chromatography, we might have many components in a sample. How do you think we can adjust for that?

By using different conditions for different analytes?

Exactly! You can dynamically adjust retention and partitioning conditions even within a single run. This is essential for mixtures with diverse components. What about the velocity of the mobile phase?

If it’s too high, the analyte won't have enough time to interact with the stationary phase!

Absolutely! You want to optimize velocity for efficient adsorption-desorption cycles. Remember, we’re balancing speed with separation efficiency.
Impacts of Modern Techniques
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

We have seen advancements like ultra-rapid chromatography that minimize analysis times greatly! Why is that important?

It allows for more samples to be processed quickly, saving time and resources!

Correct! Efficiency is key in chromatography today. Lastly, let’s consider the types of gases used in gas chromatography and how they influence separations.

Is it true that nitrogen, helium, and argon are commonly used as mobile phases?

Yes! And adjusting their flow rates can also dynamically impact separation outcomes. Great discussion, everyone! Let's summarize today's points before we wrap up.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section explains how variables like partition constants, temperature, and flow rates affect separation in chromatography. It emphasizes the dynamic nature of separation processes and the need for optimal conditions to achieve effective results, while touching on the practicalities and limitations of stationary and mobile phases.
Detailed
Detailed Summary
This section, Factors Affecting Separation, delves into the important aspects of chromatography, particularly focusing on how separations are influenced by partition constants, temperature, stationary phases, and flow rates within the chromatography system.
Key Points:
- Chromatography Basics: At the core of chromatography, the stationary phase (the column) works in tandem with the mobile phase to achieve sample separation based on differential affinities. The extent of separation is determined by the partition constant, K, between these two phases.
- Partition Constant (K): Higher values of K indicate greater retention in the column, while lower values lead to quicker elution of compounds. Manipulating factors such as temperature affects K—higher temperatures generally decrease retention time by lowering K.
- Stationary Phase Choices: Altering the stationary phase can be complex and costly. Ideally, stationary phases that target specific analytes should be selected based on the compounds of interest.
- Dynamic Adjustments: The need for dynamic conditions arises when dealing with complex mixtures containing multiple analytes. Adaptations may involve adjusting retention time and partitioning conditions during a single sample run.
- Velocity Impact: The velocity of the mobile phase affects the adsorption-desorption processes within the column, impacting overall separation efficiency. A balance must be struck between flow rate and sufficient time for analytes to interact with the stationary phase.
- Modern Methods: Advances such as ultra-rapid chromatography aim to reduce analysis time without compromising separation quality, thereby increasing sample throughput and cost-effectiveness.
- Gas Chromatography Considerations: In gas chromatography, carrier gas choices and temperature programming play significant roles in the efficiency of separations. Specific types of detectors are utilized to measure responses coming from the column based on cleavage characteristics of different analytes.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
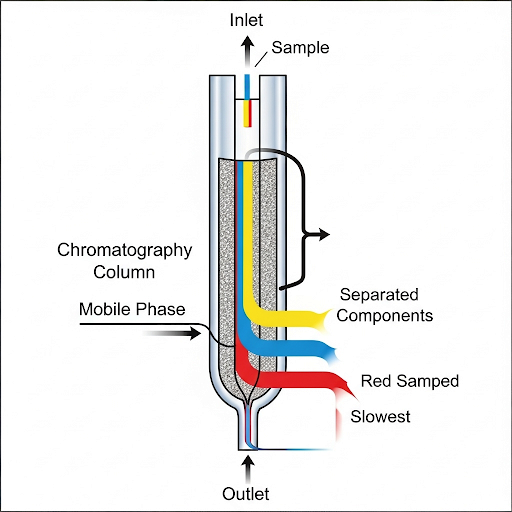
Understanding the Partition Constant (K)
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So the separation occurs mainly because it takes advantage of different affinities of the analyte between the stationary phase and mobile phase. So, in other words, we are talking about some partition constant between the stationary phase and the mobile phase. So, the extent of the separation depends on the type of the affinity.
Detailed Explanation
The partition constant, denoted as K, describes how a substance (the analyte) interacts with the two phases in chromatography: the stationary phase (the fixed phase of the column) and the mobile phase (the fluid that moves through the column). A higher K value indicates that the analyte prefers the stationary phase, leading to longer retention time in the column. Conversely, a lower K value means the analyte is more soluble in the mobile phase and exits the column more quickly. Thus, manipulating K is crucial for effective separation of components in a mixture.
Examples & Analogies
Think of the partition constant like a dance partner preference. If a dancer (analyte) prefers to stay with the dance partner (stationary phase), they'll remain on the dance floor longer (high K). If they prefer to leave the dance floor and mingle with others (mobile phase), they'll exit quickly (low K). Adjusting the conditions of the dance floor (like temperature) can change whom the dancer prefers.
Factors Impacting the Partition Constant
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
One way is the temperature, Adjust temperature factor so typically a high temperature leads to low K means low retention so higher compound comes off quickly.
Detailed Explanation
Temperature is a critical factor influencing the partition constant. Increasing the temperature generally decreases K, which leads to lower retention times for analytes. This happens because higher temperatures can increase the kinetic energy of the molecules, causing them to interact less strongly with the stationary phase. Thus, they pass through the column more rapidly, facilitating faster separations.
Examples & Analogies
Imagine a busy restaurant kitchen. On hot days, chefs (analytes) are moving around rapidly due to high energy, completing their tasks quicker (lower retention). On cooler days, they move sluggishly, taking longer to finish their dishes (higher retention). The temperature of the kitchen directly affects how fast they work.
Changing the Stationary Phase
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Second is to change the stationary phase this is far more difficult to do because stationary phases sometimes are very expensive.
Detailed Explanation
Altering the stationary phase is another method to influence separation. Different stationary phases have varying affinities for analytes, which can significantly impact the efficiency of separation. However, this option is less practical due to cost and availability. Stationary phases can be expensive and may require specific preparation, limiting their flexibility in applications.
Examples & Analogies
Consider how people get different service at restaurants based on the type of cuisine. Just as a fancy French restaurant offers different dishes and experiences compared to a diner, shifting to a different stationary phase can provide distinct separation characteristics, but not everyone can afford the fancy restaurant every time.
Changing the Mobile Phase
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
And the last class we talked a little bit about this, the most generic column are some samples columns that would do a very wide range of separations. So, the third thing that you can do is change mobile phase.
Detailed Explanation
Changing the mobile phase is the third method to influence separation. The mobile phase can affect how analytes interact during the separation process. For example, switching from water to acetonitrile as a solvent can change the polarity of the mobile phase, thereby impacting the overall retention behavior of different analytes. This allows chemists to optimize separation based on the nature of the compounds being analyzed.
Examples & Analogies
Think of a paint palette. Changing from a water-based medium to an oil-based medium can completely alter how colors mix and appear. In chromatography, just like with paint, choosing different solvents can change how components behave, enhancing clarity in separation.
Velocity and Flow Rate
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The second factor that you can play around is the velocity. So, this velocity and flow rate are components that will influence because you to realize that both components that is partition constant and velocity flow rate influences adsorption, desorption cycles rates.
Detailed Explanation
Velocity and flow rate are crucial parameters affecting the efficiency of separation in chromatography. A higher flow rate can lead to faster analysis times, but this risks not allowing enough time for analytes to interact with the stationary phase, potentially leading to poor separations. Thus, there’s a balance to strike between the speed of analysis and the quality of separation.
Examples & Analogies
Imagine a train on a track. If the train goes too fast, it might not stop at the right station (poor separation), but if it goes too slow, the journey takes longer than necessary. The train's speed is like the flow rate in chromatography—finding the right speed is key to an efficient and effective journey.
Balancing Efficiency and Analysis Time
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, you have 100 samples and I want to analyze them in a system I cannot wait for a long time for analysis to finish.
Detailed Explanation
In practical scenarios, there is often a need to balance speed and separation quality. Analysts usually have multiple samples to run and must optimize conditions to achieve efficient runs without sacrificing quality. This balance is crucial for economic reasons and is an ongoing area of development in chromatography systems.
Examples & Analogies
Consider a chef preparing meals for a large dinner party. They need to coordinate cooking times and techniques to serve all dishes at the right moment without making guests wait too long. In chromatography, optimizing separation conditions is similar—efficiency and effectiveness must harmonize to achieve the desired results.
Key Concepts
-
Partition Constant (K): Indicates the relative retention of a compound between stationary and mobile phases.
-
Retention Time: The specific time a compound takes to travel through the column.
-
Dynamic Conditions: The adjustments made during a run to optimize the separation of different analytes.
Examples & Applications
For example, if a compound has a higher partition constant, it will be retained longer in the stationary phase, leading to greater separation.
Increasing the temperature in a chromatography run can lead to lower retention times, making components exit the column more quickly.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
High K means slow; low K means go!
Stories
Imagine a highway where cars with high fuel efficiency take longer routes (high K), while sporty cars dash swiftly on direct paths (low K).
Memory Tools
Remember 'KITE' - K for constant, I for interactions, T for temperature effects, and E for elution speed.
Acronyms
PIE
Partition constant
Interaction strength
Elution kinetics!
Flash Cards
Glossary
- Chromatography
A technique used to separate mixtures based on different affinities of components for stationary and mobile phases.
- Partition Constant (K)
A ratio indicating the retention and elution behavior of a compound within a stationary and mobile phase.
- Stationary Phase
The phase that remains fixed in the chromatography column during separation.
- Mobile Phase
The phase that transports the analytes through the stationary phase.
- Retention Time
The time it takes for a compound to travel through the column and be detected.
Reference links
Supplementary resources to enhance your learning experience.
