Handling False Negatives and Positives
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding False Negatives
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’ll discuss false negatives in chemical analysis. Can anyone tell me what a false negative means?

Is it when we think a pollutant isn't there, but it actually is?

Exactly! False negatives lead us to believe there’s no issue when a problem exists. Why is this concerning in environmental analysis?

It could lead to wrong decisions that affect public health or the environment!

Correct! And what factors could lead to false negatives during sampling?

I think it can happen during transport or if there are reactions with other substances in the sample.

Right! Volatilization, reaction, and adsorption are the key processes responsible for analyte losses. Remember the acronym 'VRA' for Volatilization, Reaction, and Adsorption!

So, if we control for those processes, we can reduce false negatives, right?

Absolutely! This ties into our QA/QC protocols. Do we remember any methods to check for these losses?

We can use laboratory control samples and matrix spikes.

Great! Always remember to assess recovery efficiency as part of our quality control measures. Let's keep diving deeper into false positives next!
Exploring False Positives
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand false negatives, let’s look at false positives. What do we mean by a false positive?

It’s when we detect something that isn't really there, right?

Yes! It can occur due to sample gain or contamination. What can cause sample gain during analysis?

Maybe from dirty equipment or carryover from previous samples?

Exactly! Contaminated apparatus and sample memory can lead to false positives. Now, how do we mitigate these risks?

By using blanks for analysis and ensuring our equipment is clean!

Correct! Always run blank analyses to establish baseline readings. We want to ensure that what we detect is accurate.

And if we find something in the blank, we might need to investigate further.

Exactly! Regular QA/QC checks are crucial. Now, let's summarize what we've learned today!
Quality Control Methods
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To effectively manage false negatives and positives, what are some common QA/QC methods we can implement?

Using laboratory control samples and matrix spikes!

Yes! We use these methods to verify our recovery efficiency. What else is important?

Running blank analyses to check for contamination.

Exactly! And what are the types of blanks we can use?

There's the method blank and the instrument blank. They help to identify where contamination might be occurring.

Perfect! Remember, a systematic approach to QA/QC will help ensure our results are accurate and reliable.

So, we summarize: we use multiple controls and blanks while carefully monitoring our methods.

Exactly! You all are doing great! Keep these concepts in mind as we move forward!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, we explore the critical aspects of handling false negatives and positives in chemical analysis. It highlights how analyte losses during various stages of sampling and processing can lead to underestimating pollutants (false negatives) or overestimating them (false positives), underscoring the significance of robust quality control (QA/QC) procedures in ensuring accurate environmental monitoring.
Detailed
Handling False Negatives and Positives
In environmental analysis, the accuracy of measurements is paramount since they directly influence decisions regarding public safety, regulatory compliance, and environmental protection. Two critical issues faced in this domain are false negatives and false positives, which can stem from various challenges in the sampling process and analytical methods.
False negatives typically arise when an analyte that is present in the sample goes undetected or is underestimated, leading to decisions based on incomplete information. This can occur due to analyte losses at different stages, including during transportation, storage, or analysis itself. Analyte loss mechanisms include:
- Volatilization - The evaporation of the sample components during transport, especially if proper airtight containers are not used.
- Reaction - Chemical reactions that can degrade the analyte over time, such as biodegradation or reactions with other constituents in the sample.
- Adsorption - Loss of analyte due to adherence to the surfaces of containers, which can occur if proper materials are not used in storage.
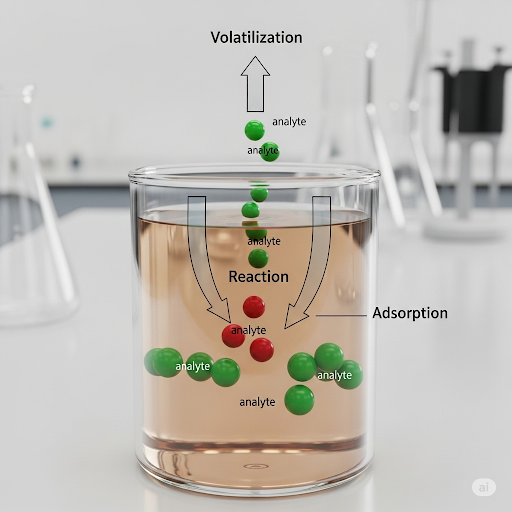
Consequently, analysts must implement stringent quality control measures to ensure the integrity of the results.
On the flip side, false positives arise from sample gains which may occur due to contamination or cross-reactions during analysis. Common scenarios include:
- Contaminated Apparatus - Using dirty glassware or contaminated analytical instruments can introduce additional analytes to the sample, skewing results.
- Sample Memory - Residues from previous samples may be inadvertently carried over into subsequent analyses, leading to misleading positive readings.
To tackle these issues, analysts frequently utilize various strategies, including:
- Laboratory Control Samples - Using known quantities of analytes to gauge recovery efficiencies.
- Matrix Spikes - Adding standards to samples to measure recovery directly and thus quantify losses induced by matrix effects.
- Blanks - Analyzing clean control samples to check for contamination and ensure proper method validation.
In summary, robust QA/QC methodologies are essential to mitigate the occurrence of false negatives and positives, thereby ensuring accurate and trustworthy environmental analyses.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding False Negatives
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Sometimes, we are talking about sample losses. There is also another aspect to sample losses lead to underestimation, which is usually falls under the category of false negative, but there is also another case of false positives, which means that what we are calling us false negative is not just whether it is not a true false answer. We are saying false negative essentially means there is an underestimation. It is another way of representing a false negative that is you are assuming that something is not there when it is there.
Detailed Explanation
A false negative occurs when a test fails to detect a substance that is actually present. For example, if a water sample is tested for a contaminant and the test results indicate that the contaminant is not present, even though it actually is, that is a false negative. This kind of mistake is especially concerning in environmental monitoring because decisions may be made based on these incorrect results.
Examples & Analogies
Think of a fire alarm that doesn't go off when there is smoke in a room. The smoke is like a contaminant in the water sample. If the alarm (the test) fails to sound, people might assume there is no fire (that the contaminant isn't there), leading to a dangerous situation.
Understanding False Positives
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Similarly, you have false positive, which means you are overestimating and this can happen if you have sample gain. So sample gain it seems not intuitive because sample will lose, where can you gain sample from since mass cannot be created from nothing. So there are a few instances where you get sample gain and the sample gain happens, the sample gain we are talking about mean by addition of the analyte from somewhere.
Detailed Explanation
A false positive occurs when a test incorrectly indicates the presence of a substance that isn’t there. In environmental analysis, this can happen due to sample gain, where contaminants from outside sources, like dirty glassware or contaminated instruments, inadvertently alter the sample's concentration. This leads to an overestimation of contaminant levels.
Examples & Analogies
Imagine you are testing soil for a specific nutrient. If you used a shovel that was previously used to dig in a fertilized area without cleaning it, you might bring some of that fertilizer into your new sample. This results in a false positive, where it seems like the new soil has a higher nutrient level than it actually does.
Sources of Sample Gain
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Addition of analyte in the sample, not at the collection site but somewhere else by an artificial mean. So how does this happen? This happens by several means. One is the most common contaminated apparatus. This is a very general case. Contaminated apparatus very simply it means dirty glassware or containers, dirty transfer equipment.
Detailed Explanation
Sample gain can occur when the equipment used in the analysis introduces contaminants unintentionally. For example, if glassware or transfer equipment is not properly cleaned, residual materials can mix with the samples, leading to erroneous high readings in contaminant levels.
Examples & Analogies
Consider cooking a recipe that calls for fresh ingredients. If you use a pot that still has residue from a previous dish without cleaning it, the dish might end up tasting completely different than intended. Similarly, dirty equipment can affect the results of the water or soil samples being tested.
Consequences of False Results
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, how do you check for this? The checking for this is why we use what is called as blanks and we have discussed this in other lecture, but the blanks analysis is very important...
Detailed Explanation
To identify and mitigate false positive results, laboratories use blank samples, which are uncontaminated samples that undergo the same analysis as actual samples. By analyzing these blanks, one can determine if there are any contaminants introduced by the equipment or the environment. If the blank shows a concentration above zero, it indicates contamination in the equipment or process.
Examples & Analogies
Think of a control group in a medical experiment. A control group does not receive the treatment being tested, allowing researchers to see how many conditions improved without intervention. If a control group experiences improvements, it indicates that other factors in the environment (like contamination) may influence results.
Methods for Ensuring Accuracy
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, to summarize we need to estimate recovery for losses, we need to do blank analysis for sample gains. Sample gain can also happen by contamination of the sample itself.
Detailed Explanation
To ensure accurate measurements in environmental analyses, recovery rates need to be established, which indicate how much of the analyte is actually recovered from the sample after processing. Additionally, systematic blank analyses help to assess contamination, ensuring that any detected samples truly represent the state of the environment rather than artifacts of the testing process.
Examples & Analogies
Imagine checking your fuel efficiency in a car. To get an accurate measurement of how far you can go on a tank of gas, you need to account for variables like driving conditions or whether the car is weighted down. Just like conducting controls (blanks) in sampling helps remove extraneous factors to ensure accuracy in test results.
Key Concepts
-
False Negative: A critical result that indicates the absence of an analyte when it is present, potentially leading to safety hazards.
-
False Positive: A misleading outcome indicating the presence of an analyte that is not truly there, which can skew data interpretation.
-
Quality Control: Essential procedures that ensure the accuracy and reliability of experiments and analyses.
-
Volatilization: A key process where analytes can evaporate, leading to potential false negatives.
-
Adsorption: Another mechanism through which analyte loss can occur by adhering to container surfaces.
Examples & Applications
A water sample collected from a river may report no pesticide presence (false negative), while the analyte has evaporated during transport due to improper handling.
During an analysis, if a dirty pipette transfers residual sulfate from a previous sample into a new sample, it could lead to a false positive indicating higher sulfate levels than present.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
To avoid false negatives, don't miss the signs, handle with care, and keep the lines fine.
Stories
Imagine a pond with fish that were missed due to evaporation during a hot day. The missed fish represent false negatives in analysis.
Memory Tools
VRA = Volatilization, Reaction, Adsorption - remember these as the key loss mechanisms.
Acronyms
Control methods
- Clean apparatus
- Run blank
- Monitor recovery.
Flash Cards
Glossary
- False Negative
A result that indicates a substance is absent when it is actually present.
- False Positive
A result that indicates a substance is present when it is actually absent.
- Quality Control (QA/QC)
Procedures implemented to ensure the accuracy and reliability of analytical results.
- Volatilization
The process of a substance changing from liquid to gas.
- Adsorption
The adhesion of molecules from a gas, liquid, or dissolved solid to a surface.
- Matrix Spike
A known quantity of analyte added to a sample to measure recovery during analysis.
- Control Sample
A sample with a known quantity of analyte used to determine recovery efficiency.
Reference links
Supplementary resources to enhance your learning experience.
