Formation of Clay Minerals
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Clay Minerals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome class! Today we’re diving into the fascinating world of clay minerals. Can anyone tell me what a mineral is?

A mineral is a natural substance that has a specific chemical composition.

So, it's different from a rock fragment which is made up of many minerals?

Exactly! A mineral is a chemical compound, whereas a rock fragment consists of multiple minerals. Most importantly, we will focus on silicate minerals as they significantly affect clay soils. Remember the term 'silicates' as they have a vast impact on soil properties.

What makes silicates so special?

Great question! The arrangement of atoms in silicate minerals leads to various silicate structures.

Can you give an example of silicate minerals?

Sure! Quartz is a common silicate mineral, but today we will focus on how these minerals relate to clay formation.
Basic Structural Units
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s move on to the basic structural units that compose these minerals: tetrahedral and octahedral units. Who can describe these units?

A tetrahedron has a central silicon atom surrounded by four oxygen atoms.

An octahedron, on the other hand, has a central ion like aluminum surrounded by hydroxyl ions.

Perfect! These units are non-neutral and thus come together to form shared sheets. What types of sheets do we see?

We have silica sheets, gibbsite sheets, and brucite sheets!

Exactly! Knowledge of these sheets is crucial for understanding clay mineral structures.
Isomorphous Substitution
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s talk about isomorphous substitution. Does anyone know what this process involves?

Isn't it when one atom in a mineral is replaced by another different atom?

Yes! Exactly. This process occurs during the formation of sheets and results in diverse clay mineral structures. Can someone give a practical example of isomorphous substitution?

Maybe when aluminum replaces silicon in some of the gibbsite structures?

Exactly right! This substitution leads to various mineral characteristics.
Types of Clay Minerals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s examine two-layer and three-layer clay minerals. Who can name a common two-layer mineral?

Kaolinite is a common two-layer clay mineral!

What about montmorillonite? Isn’t that a three-layer mineral?

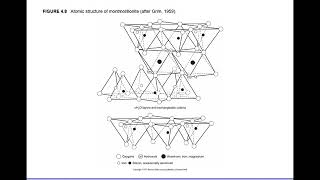
Exactly! Montmorillonite has a three-layer sheet structure and allows water to penetrate, leading to swelling. Can anyone tell me about halloysite?

Halloysite also has two layers but has water between the sheets, right?

Yes! Understanding the differences in layer structure helps us appreciate how they behave in soil.
Fine Soil Fabric
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s wrap up with fine soil fabric. Can anyone explain what this term means?

It refers to the arrangement of particles within a soil mass!

I see, and the way clay particles are arranged affects water retention, right?

Exactly! The layered and flaky structure of clay particles, along with their high surface area, allows them to hold water effectively.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Clay minerals are formed from soil particles that can be either minerals or rock fragments. Silicate minerals are particularly significant as they influence clay soil properties. The section discusses basic structural units, types of sheets formed, and the process of isomorphous substitution in clay minerals.
Detailed
Detailed Summary
This section details the formation of clay minerals from soil particles, which may either consist of individual minerals or combinations of rock fragments. It highlights that minerals are classified into categories such as silicates, aluminates, oxides, carbonates, and phosphates, with silicates being the most crucial for influencing clay soil properties.
Basic Structural Units
The text focuses on two fundamental structural units that contribute to the formation of soil minerals: tetrahedrals and octahedrals. Due to the ionic charges of these units, they do not exist independently and combine to form sheets. The interaction between tetrahedral and octahedral units leads to the development of three types of sheets: silica sheets, gibbsite sheets, and brucite sheets. An important process is isomorphous substitution, where one atom in the tetrahedral or octahedral unit is replaced by another atom, contributing to diversity in clay mineral structures.
The sheets form layered structures, resulting in two-layer and three-layer clay minerals, which are key to understanding the physical properties of clay, including their high surface area due to the plate-like formation of stacked units.
The specifics of two-layer sheet minerals such as kaolinite and halloysite, as well as three-layer clay minerals like montmorillonite and illite, are discussed, including their unique bonding characteristics and behaviors, such as swelling and water retention in clay soils. This information underscores the relationships had between clay microstructures and their implications for soil health and utilization.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Soil Particles
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A soil particle may be a mineral or a rock fragment. A mineral is a chemical compound formed in nature during a geological process, whereas a rock fragment has a combination of one or more minerals.
Detailed Explanation
Soil particles are classified into two main categories: minerals and rock fragments. Minerals are pure chemical compounds that naturally form through geological processes, meaning they are created from natural sources over time. In contrast, rock fragments are not pure substances; instead, they are made up of one or more different minerals combined together.
Examples & Analogies
Think of a mineral like a single ingredient in a recipe, such as flour, while a rock fragment is like a cake made from various ingredients mixed together. Just as a cake is made from different components, a rock fragment contains multiple minerals.
Classification of Minerals
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Based on the nature of atoms, minerals are classified as silicates, aluminates, oxides, carbonates and phosphates. Out of these, silicate minerals are the most important as they influence the properties of clay soils.
Detailed Explanation
Minerals can be categorized based on the types of atoms they contain. There are five main classifications: silicates, aluminates, oxides, carbonates, and phosphates. Among these, silicate minerals are particularly significant because they play a crucial role in determining the characteristics of clay soils, such as their texture and nutrient-holding capacity.
Examples & Analogies
Consider silicate minerals as the foundation of a building; just as a solid base supports the entire structure, silicate minerals are essential for the qualities of clay soils. If the foundation is strong and reliable, the building will stand firm.
Basic Structural Units
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Soil minerals are formed from two basic structural units: tetrahedral and octahedral. Considering the valencies of the atoms forming the units, it is clear that the units are not electrically neutral and as such do not exist as single units.
Detailed Explanation
Soil minerals consist of two primary building blocks: the tetrahedral unit and the octahedral unit. The tetrahedral unit is shaped like a pyramid with a silicon atom at its center surrounded by four oxygen atoms. The octahedral unit, on the other hand, features an aluminum or magnesium ion surrounded by six hydroxide ions. These units are not electrically balanced, meaning they cannot stand alone and instead combine to form more complex structures.
Examples & Analogies
Imagine building blocks made of distinct shapes—like tetrahedrons and octahedrons. Alone, they don’t hold together well, but when combined into larger structures, such as a fortress or castle, they create a strong and stable assembly.
Formation of Sheets
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The basic units combine to form sheets in which the oxygen or hydroxyl ions are shared among adjacent units. Three types of sheets are thus formed, namely silica sheet, gibbsite sheet and brucite sheet.
Detailed Explanation
The tetrahedral and octahedral units connect to form layers called sheets. In these sheets, oxygen or hydroxyl ions are shared between adjacent units, leading to three specific types of sheets: the silica sheet, the gibbsite sheet, and the brucite sheet. These sheets are essential in the formation of clay minerals as they create a layered structure that enhances the properties of the soil.
Examples & Analogies
Consider stacking sheets of paper in a binder. Each sheet is like a layer of minerals, and as you pile more on top, they become increasingly stable and organized, just as the sheets in soil help stabilize and structure the overall material.
Isomorphous Substitution
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Isomorphous substitution is the replacement of the central atom of the tetrahedral or octahedral unit by another atom during the formation of the sheets.
Detailed Explanation
Isomorphous substitution occurs when one atom in the tetrahedral or octahedral unit is replaced by another atom that is similar in size and charge. This substitution can significantly affect the properties of the mineral. For example, replacing some silicon in the tetrahedral unit with aluminum can create variations in the mineral's characteristics, impacting how it interacts with water and other elements.
Examples & Analogies
Think of isomorphous substitution like swapping one ingredient for another in a recipe—if you use honey instead of sugar, the end result will still be sweet, but the flavor can change. This is similar to how minerals can still function but exhibit different properties based on the atom substitutions.
Formation of Clay Minerals
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The sheets then combine to form various two-layer or three-layer sheet minerals. As the basic units of clay minerals are sheet-like structures, the particle formed from stacking of the basic units is also plate-like. As a result, the surface area per unit mass becomes very large.
Detailed Explanation
The sheets formed from the basic units come together to create either two-layer or three-layer mineral structures. The way these layers stack results in particles that resemble thin plates. This plate-like shape increases the surface area of the particles significantly, allowing clay minerals to retain moisture and nutrients effectively.
Examples & Analogies
You can think of it like piling thin pancakes—each pancake represents a layer of clay minerals. When you stack them up, you create a tall stack (high surface area) that can absorb syrup (water and nutrients) much better than a single thick pancake.
Key Concepts
-
Formation of Clay Minerals: Clay minerals arise from soil particles composed of either minerals or rock fragments, significantly impacting soil properties.
-
Basic Structural Units: The two main structural components of clay minerals are tetrahedral and octahedral units.
-
Isomorphous Substitution: This process helps in forming a variety of clay minerals through the replacement of atoms in the crystal structure.
-
Types of Clay Minerals: Key examples include kaolinite and montmorillonite, both of which exhibit distinct behaviors concerning water.
-
Fine Soil Fabric: The arrangement of soil particles influences physical properties, including water retention and soil stability.
Examples & Applications
Kaolinite is a stable two-layer clay mineral, often found in residual clay deposits, which does not swell.
Montmorillonite is a three-layer clay mineral that can absorb large amounts of water and leads to swelling during wet conditions.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Tetrahedrons crunch, silicate munch, from layers so fine, water flows in line.
Stories
Once upon a time in the soil kingdom, the tetrahedral units danced with the octahedral units to form strong, stable structures that helped crops grow. Their friendship was built on layers of trust, and together they kept the water safe and sound.
Memory Tools
Remember 'T.O.S.' for the types of sheets: Tetrahedral (T), Octahedral (O), and Silica (S).
Acronyms
K.M.M. for clay minerals
Kaolinite (K)
Montmorillonite (M)
and Illite (M).
Flash Cards
Glossary
- Mineral
A naturally occurring chemical compound with a specific chemical composition.
- Rock Fragment
A piece of a rock that contains combinations of one or more minerals.
- Silicate
A mineral group that contains silicon and oxygen, important for clay soil properties.
- Tetrahedral Unit
A basic structural unit made of a central silicon atom surrounded by four oxygen atoms.
- Octahedral Unit
A basic structural unit made of a central aluminum or magnesium ion surrounded by six hydroxyl ions.
- Isomorphous Substitution
The process where one atom in a mineral is replaced by another atom in the crystal structure.
- Sheet Minerals
Minerals that are constructed from stacked sheets formed by tetrahedral and octahedral units.
- Kaolinite
A common two-layer clay mineral that is stable and does not swell.
- Montmorillonite
A three-layer clay mineral that swells when water is absorbed.
- Fine Soil Fabric
The arrangement and organization of soil particles within a soil mass.
Reference links
Supplementary resources to enhance your learning experience.
