Structure of Clay Minerals
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Basic Structural Units
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will discuss the basic structural units that form clay minerals. Can anyone tell me what these units are?

Are they tetrahedral and octahedral units?

Correct, Student_1! The tetrahedral unit has a silicon atom at its center surrounded by four oxygen atoms. This forms a tetrahedron shape. What about the octahedral unit?

The octahedral unit has aluminum or magnesium at the center, surrounded by six hydroxyl groups?

Exactly! Combining these units creates various types of clay structures, which we will explore shortly. Remember: 'Tetrahedrons team up with octahedrons to build our clay!'

Can you remind us what is meant by the unit not being electrically neutral?

Great question! Since these units are not neutral, they interact to form sheets. The bond between the units shapes the overall characteristics of the clay minerals. Let's move to what types of sheets are formed from these units.

Are there specific types of sheets?

Yes! We have the silica sheet, gibbsite sheet, and brucite sheet, all formed through the sharing of oxygen or hydroxyl ions. By the end of this class, you'll know how they affect clay mineral properties!

To summarize, today we learned about tetrahedral and octahedral units which combine to form sheets in clay minerals.
Formation of Sheet Minerals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand the basic units, let's talk about how these combine to form actual clay minerals like kaolinite and montmorillonite. Can anyone describe kaolinite?

Kaolinite is formed by stacking a gibbsite sheet on a silica sheet, right?

Exactly, Student_1! The gibbsite and silica sheets are bonded through hydrogen bonds which contribute to the stable structure of kaolinite. What can you tell me about the water ability of kaolinite?

It does not absorb much water, because the strong bonds prevent water from entering.

Exactly! Now, what about montmorillonite?

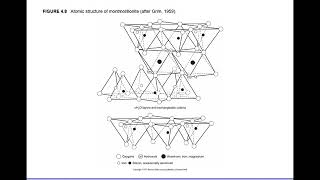
Montmorillonite has a three-layer structure, with silica on both sides of a gibbsite sheet.

Correct! And this weak bond allows water to enter, causing swelling. Remember: 'Montmorillonite makes a mountain of moisture!' Can someone tell me what happens during dry weather?

It shrinks because of the water loss!

Great observation! Thus, understanding these minerals helps us manage agricultural soil better. To wrap up, kaolinite is stable while montmorillonite is flexible but can swell or shrink.
Illite and Fine Soil Fabric
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will discuss Illite. Who can remind me what it has in common with Montmorillonite?

Illite consists of montmorillonite units, but it's more stable, right?

Correct! It's bonded by secondary valence forces and potassium ions. Can anyone tell me what happens to aluminum in Illite?

There's about a 20% replacement of aluminum with silicon due to isomorphic substitution.

Exactly! This isomorphous substitution is crucial. Now let's shift gears and talk about soil fabric. Who can explain what fine soil fabric means?

It's the arrangement and organization of particles within the soil mass.

Good! Clay particles are flaky and very thin compared to their length. This gives them a high surface area. Any thoughts on why this is important?

It helps hold a lot of water due to the charge they carry!

Correct! Clay’s ability to attract positive ions is essential for soil health. To conclude, we've learned about Illite's structure and the importance of soil fabric.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section discusses how clay minerals are composed of tetrahedral and octahedral structural units that create sheets, leading to different mineral types including kaolinite, halloysite, montmorillonite, and illite. These arrangements significantly affect the physical and chemical properties of clay soils.
Detailed
Structure of Clay Minerals
Clay minerals are foundational components of clay soils, formed from tetrahedral and octahedral structural units. The tetrahedral unit consists of a silicon atom surrounded by four oxygen atoms, forming a silica sheet when combined with other tetrahedrons. The octahedral unit, on the other hand, is built around a central aluminum or magnesium atom surrounded by six hydroxyl ions, leading to the creation of gibbsite and brucite sheets.
Two-layer and three-layer sheet minerals arise from these basic units, with notable types being kaolinite (two-layer) and montmorillonite (three-layer). Kaolinite forms a stable lattice held by hydrogen bonds, preventing water infiltration, while montmorillonite is less stable due to weak van der Waals forces, resulting in significant water absorption and shrinkage during dry conditions. Illite, deriving from montmorillonite, exhibits stability without the swelling characteristic of montmorillonite.
This structural diversity contributes to the properties of clay soils, such as their high surface area and ability to retain water, which are critical for agricultural and environmental applications.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Tetrahedral Unit
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A tetrahedral unit consists of a central silicon atom that is surrounded by four oxygen atoms located at the corners of a tetrahedron. A combination of tetrahedrons forms a silica sheet.
Detailed Explanation
A tetrahedral unit is a structural component of clay minerals. It has a central silicon atom surrounded by four oxygen atoms, forming a shape known as a tetrahedron. When multiple tetrahedral units come together, they create a larger structure called a silica sheet. This arrangement is fundamental in the formation of various clay minerals due to the way they bond and stack together.
Examples & Analogies
Imagine building a model using tetrahedron-shaped building blocks. Each block represents a tetrahedral unit, and when you connect several of them together, they create a flat sheet, similar to how silica sheets are formed in clay minerals.
Octahedral Unit
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
An octahedral unit consists of a central ion, either aluminium or magnesium, that is surrounded by six hydroxyl ions located at the corners of an octahedron. A combination of aluminium-hydroxyl octahedrons forms a gibbsite sheet, whereas a combination of magnesium-hydroxyl octahedrons forms a brucite sheet.
Detailed Explanation
The octahedral unit is another essential structural component of clay minerals. In this unit, a central ion (typically aluminium or magnesium) is surrounded by six hydroxyl ions, forming an octahedron. Just like the tetrahedrons in silica sheets, multiple octahedral units can combine to form larger structures—gibbsite sheets from aluminium-hydroxyl octahedrons and brucite sheets from magnesium-hydroxyl octahedrons.
Examples & Analogies
Think of the octahedral unit like a crowded square where a central figure (the central ion) is surrounded by six friends (the hydroxyl ions) standing at the corners of the square. This group can then form their own circle (the gibbsite or brucite sheets) when they come together.
Combination of Sheets
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The sheets then combine to form various two-layer or three-layer sheet minerals.
Detailed Explanation
Now that we have silica sheets and gibbsite or brucite sheets, these sheets can combine with each other to form more complex structures. When a silica sheet combines with a gibbsite sheet, it creates a two-layer sheet mineral. Similarly, when different combinations occur, we can create three-layer sheet minerals. These arrangements play a significant role in the physical and chemical properties of clay minerals.
Examples & Analogies
Imagine layering your favorite sandwiches: if you place two slices of bread (the silica and gibbsite sheets) on top of each other, you have a two-layer sandwich. If you add another layer, you create a three-layer sandwich that’s even more complex and interesting!
Layered Structures and Surface Area
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
As the basic units of clay minerals are sheet-like structures, the particle formed from stacking of the basic units is also plate-like. As a result, the surface area per unit mass becomes very large.
Detailed Explanation
The stacking arrangement of these sheets gives clay minerals their unique plate-like shape. This structure significantly increases the surface area of the particles, meaning there is a much larger surface available for interactions, such as retaining water and nutrients. This property affects how clay behaves in soil and its ability to hold moisture.
Examples & Analogies
Think of a stack of thin, oversized pancakes. Even though each pancake (the sheet) is thin, when you stack them up, you have a large area of pancake available to soak up syrup (water and nutrients). This demonstrates why clay's structure is crucial for soil properties.
Isomorphous Substitution
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Isomorphous substitution is the replacement of the central atom of the tetrahedral or octahedral unit by another atom during the formation of the sheets.
Detailed Explanation
Isomorphous substitution is a process where one atom in the structure is replaced by another atom of similar size but different charge. This can happen in the tetrahedral or octahedral units, leading to changes in the mineral's properties, such as its behavior in soil. This substitution can affect characteristics like ion exchange capacity, which is important for soil fertility.
Examples & Analogies
Imagine substituting one fruit for another in a fruit salad. If you swap out strawberries (a silicon atom) for blueberries (another atom), the overall taste and appearance of the salad (the mineral) changes, but it’s still a fruit salad. Similarly, the clay mineral retains its identity while having different properties due to the substitution.
Key Concepts
-
Tetrahedral Unit: The basic unit of clay minerals composed of silicon and oxygen.
-
Octahedral Unit: Another key unit that consists of aluminum or magnesium surrounded by hydroxyl groups.
-
Sheet Structures: Formed by the combination of tetrahedral and octahedral units, leading to various clay minerals.
-
Kaolinite: A type of clay mineral characterized by its stability and low water absorption.
-
Montmorillonite: A clay mineral noted for its ability to swell and shrink due to water absorption.
-
Illite: A stable clay mineral that retains its structure without significant swelling.
-
Soil Fabric: The arrangement and organization of soil particles influencing their properties.
Examples & Applications
Kaolinite is often found in residual soil deposits and is utilized in ceramics due to its stable structure.
Montmorillonite is used in drilling fluids for its capacity to absorb large volumes of water.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Tetra and octa in harmony, build clay minerals for you and me!
Stories
Once upon a time, in a land of clay, tetrahedrons and octahedrons danced all day! Together they formed sheets, so pretty and neat, and soon they made minerals that couldn't be beat.
Memory Tools
Remember 'T-O-N' for Tetra, Octa, and the Natural elements they form together.
Acronyms
T.O.C. - Tetrahedral, Octahedral, Clay minerals!
Flash Cards
Glossary
- Tetrahedral Unit
A structural component of clay minerals consisting of a silicon atom surrounded by four oxygen atoms.
- Octahedral Unit
A structural component formed by a central ion, either aluminum or magnesium, surrounded by six hydroxyl ions.
- Isomorphous Substitution
The replacement of one atom in a mineral structure by another atom of similar size and charge.
- Silica Sheet
A flat structure formed by combining tetrahedral units.
- Gibbsite Sheet
A structure formed from aluminum-hydroxyl octahedrons.
- Brucite Sheet
A structure formed from magnesium-hydroxyl octahedrons.
- Kaolinite
A type of clay mineral formed by stacking gibbsite and silica sheets, stable and not expandable.
- Montmorillonite
A clay mineral formed from three-layer sheets, allowing significant water absorption.
- Illite
A stable clay mineral that does not swell or shrink.
- Soil Fabric
The arrangement and organization of soil particles within a soil mass.
Reference links
Supplementary resources to enhance your learning experience.
