Two-layer Sheet Minerals
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Basic Structural Units
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

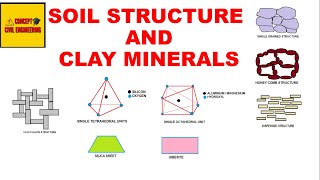
Let's start by discussing the basic structural units of clay minerals. Can anyone tell me what makes up a tetrahedral unit?

Is it formed by silicon and oxygen atoms?

That's correct! A tetrahedral unit has a central silicon atom surrounded by four oxygen atoms. Now, who can tell me about the octahedral unit?

It's made up of either aluminium or magnesium surrounded by hydroxyl ions, right?

Exactly! These units combine to form sheets in clay minerals. Remember, you can think of tetrahedrons like three-dimensional pyramids. Now, let's remember this with the mnemonic 'Silly Tetra' – for Silicon and four Oxygen at the corners.
Formation of Two-layer Sheet Minerals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let's dive into two-layer sheet minerals specifically keolinite and halloysite. Student_3, can you explain how kaolinite is structured?

Kaolinite is formed by stacking gibbsite sheets on top of silica sheets.

Correct! And it's important to note that these sheets are held together by hydrogen bonds, which makes kaolinite very stable. Who can discuss halloysite?

Halloysite has a similar structure but contains water between the sheets.

Good observation! This water presence impacts the behavior of halloysite in wet conditions. To help remember these concepts, think of 'Kaolin is Stable' for kaolinite and 'H2O between layers' for halloysite.
Comparative Properties
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's compare the properties of our two-layer minerals with montmorillonite. What can you tell me about the bonding in montmorillonite, Student_1?

Montmorillonite has three-layer units and is bonded by van der Waals forces, which are weaker than kaolinite's hydrogen bonds?

That's right! This weaker bonding allows for greater water absorption, leading to swelling. A great mnemonic is 'Mighty Montmorillonite Munches Moisture' to remember its water-loving property!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section explores two-layer sheet minerals, focusing on kaolinite and halloysite, explaining their structures formed from tetrahedral and octahedral units. It highlights the bonding characteristics and physical properties, including the stability of these minerals in various environmental contexts.
Detailed
Detailed Summary
The formation of clay minerals, particularly two-layer sheet minerals, is fundamental to understanding soil composition and health. This section specifically examines kaolinite and halloysite, two prominent minerals formed from basic structural units known as tetrahedral and octahedral units. A tetrahedral unit comprises a central silicon atom surrounded by four oxygen atoms, forming a silica sheet, while an octahedral unit consists of aluminium or magnesium surrounded by six hydroxyl ions, contributing to gibbsite and brucite sheets.
Kaolinite is characterized by its stable two-layer unit that stacks gibbsite sheets atop silica sheets, held together tightly by hydrogen bonds. This strong attraction prevents water infiltration, making kaolinite particularly stable and resistant to expansion when saturated. It is notable as the most common residual clay. Conversely, halloysite also features a two-layer structure but incorporates water between the sheets, displaying varied properties and behaviors in different moisture conditions.
In contrast, other clay minerals like montmorillonite and illite exhibit more complex structures with three-layer configurations. The understanding of these minerals not only informs soil properties but also assists in agricultural and environmental applications.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Kaolinite Mineral
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The basic kaolinite unit is a two-layer unit that is formed by stacking a gibbsite sheet on a silica sheet. These basic units are then stacked one on top of the other to form a lattice of the mineral. The units are held together by hydrogen bonds. The strong bonding does not permit water to enter the lattice. Thus, kaolinite minerals are stable and do not expand under saturation. Kaolinite is the most abundant constituent of residual clay deposits.
Detailed Explanation
Kaolinite is a key two-layer mineral made by layering a gibbsite sheet over a silica sheet. The gibbsite sheet contains aluminum and hydroxyl ions, while the silica sheet consists of silicon and oxygen. When stacked, these layers form a stable lattice structure held together by hydrogen bonds. Due to this strong bonding, water cannot easily penetrate, so kaolinite does not swell or shrink when wet, making it very stable. This stability is why kaolinite is prevalent in residual clay deposits, meaning soil that remains after the underlying rock has weathered.
Examples & Analogies
Think of kaolinite like a sturdy sandwich made with strong bread (the silica sheet) holding together a delicious filling (the gibbsite sheet). The sandwich's tight bonding makes it difficult for ingredients (water) to seep through, ensuring it stays intact and doesn’t become soggy!
Halloysite Mineral
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The basic unit is also a two-layer sheet similar to that of kaolinite except for the presence of water between the sheets.
Detailed Explanation
Halloysite is another two-layer mineral similar to kaolinite, but with a significant difference: it contains water molecules trapped between the layers of the gibbsite and silica sheets. This water can cause the halloysite to behave differently in soils, allowing it to expand and contract when it gets wet or dries out. Even though it retains some stability, the presence of water can influence its overall properties and behavior in soil.
Examples & Analogies
Imagine halloysite as a sponge sandwich where every layer has a little water trapped inside. When you squeeze the sandwich, it changes shape slightly. This is how halloysite can expand and contract with moisture, much like how a sponge absorbs water!
Key Concepts
-
Tetrahedral Unit: A structural component made of silicon and oxygen found in clay minerals.
-
Octahedral Unit: A structural component made of aluminium or magnesium surrounded by hydroxyl ions in clay minerals.
-
Two-layer Sheet Minerals: Minerals like kaolinite and halloysite formed from stacking sheets of tetrahedral and octahedral units.
-
Hydrogen Bonding in Kaolinite: Strong bonding that keeps kaolinite stable and prevents water infiltration.
-
Water Absorption in Halloysite: The presence of water between sheets impacts halloysite's volume and stability.
Examples & Applications
Kaolinite is commonly found in residual soils and is used in pottery due to its stable structure.
Halloysite can exhibit different properties based on moisture levels, affecting its behavior in natural environments.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In soil so clay, the sheets do lay,
Acronyms
K-H-S
Kaolinite Holds Steady; Halloysite has Water.
Stories
Imagine a strong house (kaolinite) built on solid ground without leaks, and a soft cottage (halloysite) with a garden hose between its walls ready to burst when wet.
Memory Tools
Think of 'K-G-H' for Kaolinite Gibbsite, Halloysite—helps remember the two-layer structure with gibbsite on top.
Flash Cards
Glossary
- Tetrahedral Unit
A structural unit in clay minerals composed of a silicon atom surrounded by four oxygen atoms.
- Octahedral Unit
A structural unit in clay minerals consisting of an aluminium or magnesium atom surrounded by six hydroxyl ions.
- Kaolinite
A type of clay mineral formed by stacking a gibbsite sheet on a silica sheet, known for its stability.
- Halloysite
A clay mineral similar to kaolinite but has water between the layers, affecting its properties.
- Montmorillonite
A clay mineral with three-layer units that bond weakly, allowing large water absorption and swelling.
- Isomorphous Substitution
The process of replacing an atom in the tetrahedral or octahedral unit with another atom without altering the crystal structure.
Reference links
Supplementary resources to enhance your learning experience.
