Three-layer Sheet Minerals
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Three-layer Sheet Minerals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are going to discuss three-layer sheet minerals, specifically montmorillonite and illite. Can anyone tell me what they understand by the term 'three-layer sheet minerals'?

I think it means they have three layers of atoms or something like that.

That's correct! These minerals consist of one gibbsite sheet sandwiched between two silica sheets. You can think of them like a sandwich! Now, who can tell me the importance of this structure?

Does this structure affect how much water they can hold?

Absolutely! The unique arrangement determines their ability to swell and take in water. This quality is crucial for soil properties.
Properties of Montmorillonite
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s dive deeper into montmorillonite. Can anyone describe how this mineral behaves when wet and dry?

I remember learning that it can swell when it absorbs water.

Exactly! The weak van der Waals forces between the layers allow it to take in large amounts of water. This is why it swells. What happens when it dries out?

It shrinks back down, right?

Correct! The dynamic behavior of montmorillonite is essential in various applications like agriculture and construction.
Understanding Illite
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss illite. How is it different from montmorillonite?

Is it more stable than montmorillonite?

Yes! Illite has stronger bonding due to potassium ions, making it less prone to swelling. Why do you think stability is important for soil?

Because it helps plants grow better if the soil doesn't change too much.

Exactly! Stability ensures that nutrients and moisture levels remain consistent, which is essential for agriculture.
Overall Implications of Three-layer Minerals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let’s summarize the importance of three-layer minerals in soil science. Why is it crucial for us to understand these minerals?

I think it helps us improve soil management and agriculture.

Right! They influence water retention and nutrient availability. Does anyone have thoughts on how this affects soil use in farming?

We can choose the right minerals for the crops we want to grow!

Exactly! Great job today everyone! Let’s remember how crucial these minerals are in soil management.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section delves into three-layer sheet minerals, particularly focusing on montmorillonite and illite. These minerals consist of alternating sheets of silica and gibbsite, which contribute to their unique properties like swelling and stability in various environmental conditions.
Detailed
Three-Layer Sheet Minerals
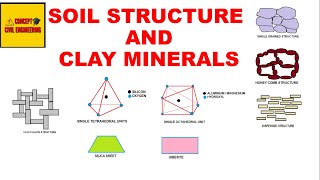
Three-layer sheet minerals, primarily montmorillonite and illite, are crucial types of clay minerals that are characterized by their distinct three-layer structure. Each unit comprises a gibbsite sheet sandwiched between two silica sheets. This layered structure is significant because it affects how these minerals interact with water, nutrients, and other soil components.
- Montmorillonite has weak van der Waals forces between layers, allowing it to imbibe water, leading to swelling when wet, and shrinkage upon drying.
- Illite, in contrast, has stronger bonding mechanisms, primarily involving potassium ions, which makes it more stable and less responsive to changes in moisture. These unique properties of three-layer sheet minerals are critical for understanding soil behavior and managing agricultural practices.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Montmorillonite Mineral
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Montmorillonite and illite clay minerals are the most common. A basic three-layer sheet unit is formed by keeping one silica sheet each on the top and at the bottom of a gibbsite sheet. These units are stacked to form a lattice as shown.
Montmorillonite Mineral:
The bonding between the three-layer units is by van der Waals forces. This bonding is very weak and water can enter easily. Thus, this mineral can imbibe a large quantity of water causing swelling. During dry weather, there will be shrinkage.
Detailed Explanation
Montmorillonite is a type of clay mineral that consists of three-layer structures, where each structure has a silica sheet positioned above and below a gibbsite sheet. The layers are bonded together with weak van der Waals forces. Because of this weak bonding, when water comes into contact with montmorillonite, it can easily enter between the layers, leading to expansion, or swelling. Conversely, when the clay dries, it contracts, or shrinks.
Examples & Analogies
Imagine a sponge that soaks up water and expands when it's wet, and shrinks when it dries. Montmorillonite works in a similar way. Just like the sponge can hold and release water, montmorillonite expands when it absorbs water and contracts when the water is removed.
Illite Mineral
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Illite consists of the basic montmorillonite units but are bonded by secondary valence forces and potassium ions, as shown. There is about 20% replacement of aluminium with silicon in the gibbsite sheet due to isomorphous substitution. This mineral is very stable and does not swell or shrink.
Detailed Explanation
Illite is another clay mineral that, while sharing similarities with montmorillonite, has a more stable structure. The basic units of illite are the same as those in montmorillonite, but in illite, the layers are held together by stronger secondary valence forces and potassium ions. This strong attraction means that illite does not swell or shrink like montmorillonite, making it a more stable mineral in varying moisture conditions.
Examples & Analogies
Think of a strong building with tightly interlocked bricks versus a temporary tent. The building (represented by illite) remains sturdy and doesn’t change shape in the weather, while the tent (which could represent montmorillonite) might expand or collapse depending on the rain. Illite, like the building, maintains its stable structure regardless of environmental changes.
Key Concepts
-
Three-Layer Structure: A structure consisting of one gibbsite sheet between two silica sheets.
-
Montmorillonite: A type of clay that expands when wet due to weak interlayer forces.
-
Illite: More stable than montmorillonite and less prone to water absorption due to strong bonding.
Examples & Applications
Montmorillonite is commonly found in soils and can cause structural changes in constructions due to its swelling behavior.
Illite is often used in agricultural fields where stable soil conditions are necessary for crop growth.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Montmorillonite swells with delight, absorbing water, day and night!
Stories
Imagine a sandwich: the strong gibbsite holds the layers of silica tight, keeping each bite flavorful and right!
Memory Tools
Mighty Montmorillonite swells while Illite is Insightful and stable!
Acronyms
MIS
Montmorillonite Is Stretchy
Illite Stays Stable!
Flash Cards
Glossary
- Montmorillonite
A type of clay mineral known for its ability to absorb water and swell, with weak bonding forces between its layers.
- Illite
A type of clay mineral characterized by its stability and less swelling due to strong bonding with potassium ions.
- Layer Structure
The arrangement of minerals characterized by stacked sheets, influencing their physical properties.
- Isomorphous Substitution
The replacement of one element in a mineral's structure with another of similar size, leading to variations in chemical composition.
Reference links
Supplementary resources to enhance your learning experience.
