Activation Energy
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
What is Activation Energy?
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we'll explore activation energy, abbreviated as Ea. Can anyone tell me what you think activation energy means?

Is it the energy needed for a reaction to start?

Exactly! Activation energy is the minimum energy that reactant molecules must possess to successfully collide and form products. This is part of what we call collision theory.

So, does a higher activation energy mean the reaction will take longer?

That's correct! A higher Ea means fewer molecules can overcome this energy barrier, leading to slower reaction rates. Think of it as a steep hill that fewer people can climb.

What happens if the activation energy is low?

Great question! If Ea is low, more molecules can easily gain enough energy to reach the transition state, resulting in faster reactions. Remember 'low Ea, quick to react’.

What does a reaction pathway look like on a graph?

Good point! On an energy diagram, you'd see reactants at one energy level, rising to a peak at the transition state, and then falling to the levels of the products. We'll visualize this shortly.

To summarize, activation energy is key to understanding the behavior of reactions in kinetic studies.
Activation Energy and Reaction Rates
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s delve deeper into how activation energy affects reaction rates. Can anyone explain how temperature might influence activation energy?

Maybe if the temperature is higher, it means more molecules have enough energy?

Exactly! As temperature increases, molecular speeds increase, allowing more molecules to reach or exceed the activation energy. This is where the Arrhenius equation comes in!

What’s the Arrhenius equation?

The Arrhenius equation, k = A * exp(-Ea/(R*T)), describes the rate constant k as dependent on activation energy Ea and temperature T. 'A' represents the pre-exponential factor, which incorporates frequency of collisions.

How does this relate to energy distributions?

Good connection! The Maxwell-Boltzmann distribution shows the range of molecular energies at a given temperature, where only those with energies surpassing Ea can react. That’s a crucial linkage!

So, is a catalyst related to activation energy?

Yes, indeed! Catalysts lower the activation energy by providing alternative pathways for the reaction, thus speeding up the rate. This is a key application in chemistry!

In summary, the relationship between activation energy, temperature, and catalysts shapes our understanding of how and why reactions proceed at particular rates.
Understanding the Energy Diagram
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s visualize activation energy through an energy diagram. Who can describe what we might see in such a diagram?

I think we will see reactants starting at one energy level and moving up to a peak.

Correct! The peak represents the transition state where the energy is highest, and then it drops down to the energy level of the products.

What do the different heights mean?

The height of the peak indicates the activation energy. A higher peak corresponds to a higher Ea, meaning a smaller number of molecules can reach it, and thus a slower reaction.

So what if the products are higher in energy than the reactants?

That describes an endothermic reaction, where the activation energy for the reverse reaction would also be important to note. The energy landscape is crucial for understanding thermodynamics and kinetics!

Can we see examples of real reactions that illustrate both high and low activation energies?

Absolutely! For example, combustion reactions typically have lower Ea due to external energy sources, while the decomposition of hydrogen peroxide has a much higher activation energy.

In summary, energy diagrams effectively illustrate the activation energy concept, helping us visualize the energy transitions that occur during chemical reactions.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section delves into the concept of activation energy (Ea), illustrating its role as a barrier that reactants must overcome to initiate a reaction. It discusses how the energy landscape of a reaction pathway influences the rates of reactions, noting that a higher activation energy correlates with slower reactions, while a lower activation energy leads to increased reaction rates.
Detailed
Activation Energy
Activation energy (Ea) is essential in understanding chemical kinetics as it defines the minimum energy required for reactants to progress to products. In terms of energy diagrams, the pathway from reactants to products typically features an energy peak known as the transition state (E‡), where the potential energy is at its highest. The difference between the energy of the transition state and the energy of the reactants defines the activation energy for the forward reaction:
- For an exothermic reaction where products have lower energy than reactants, the activation energy for the reverse reaction is also noted.
- Activation energy has profound implications for the reaction rate: higher Ea means fewer molecules have sufficient energy to react, leading to slower rates, while lower Ea increases molecular collisions that can lead to product formation.
- This section emphasizes the connections between molecular energy distributions depicted by the Maxwell-Boltzmann distribution, the Arrhenius equation, and how these concepts apply to rate laws and reaction mechanisms.
Understanding activation energy is critical for areas including catalysis, where catalysts lower the activation energy, enhancing reaction rates without being consumed.
Youtube Videos
Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Activation Energy
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The activation energy, Ea, is the minimum energy barrier that reactant molecules must overcome to form products.
Detailed Explanation
Activation energy represents the energy threshold needed for a reaction to occur. Think of it as a hurdle that reactant particles need to jump over to transform into products. If the energy of the reactants is below this threshold, the reaction won't happen, no matter how often the particles collide.
Examples & Analogies
Imagine trying to roll a stone over a hill. If the hill is too tall (high activation energy), the stone won't reach the other side. Only when you push the stone with enough force (providing the required energy) can it roll over and continue down the other side (forming products).
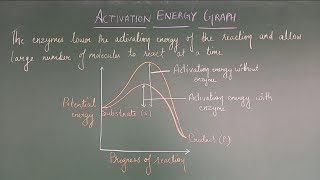
Potential Energy Diagram
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
On a diagram of potential energy versus reaction progress (reaction coordinate):
1. Reactants start at energy E_reactants.
2. As they approach each other and bonds start to rearrange, energy rises until the system reaches the transition state at energy E‡ (pronounced “E double dagger”).
3. After passing the transition state, energy falls to the level of the products, E_products.
Detailed Explanation
A potential energy diagram visualizes the energy changes during a chemical reaction. Initially, reactants have a certain energy level. As the reactants collide and begin to react, their energy increases until it peaks at the transition state. This highest point represents the activation energy that must be overcome for the reaction to proceed. Once this energy barrier is surpassed, the system loses energy and stabilizes into the products.
Examples & Analogies
Consider climbing a steep hill (the energy peak at the transition state) while hiking. You need to exert effort (overcome energy barrier) to reach the top. Once there, you can enjoy a smooth slope down to the valley (the products).
Forward and Reverse Activation Energy
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The activation energy for the forward reaction is
Ea_forward = E‡ – E_reactants.
If the reaction is exothermic (products lower in energy than reactants), the activation energy for the reverse reaction is
Ea_reverse = E‡ – E_products = Ea_forward + |Delta H°|.
Detailed Explanation
Activation energies can differ depending on the direction of the reaction. For a forward reaction, the energy needed is determined by the difference between the transition state and the starting energy of the reactants. In exothermic reactions, the reverse process has a higher energy barrier compared to going forward because the products are at a lower energy level. The change in enthalpy (Delta H°) essentially adds to the energy needed to start the reverse reaction.
Examples & Analogies
Think of a seesaw. When one side is raised (the forward reaction), it’s easier to let it drop (less energy needed to go down) than to lift it back up from the ground (the reverse reaction) since it has to overcome that height difference.
Effect of Activation Energy on Reaction Rate
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A larger Ea means that at a given temperature, fewer molecules have enough energy to cross the barrier, so the reaction is slower. A smaller Ea corresponds to a faster reaction.
Detailed Explanation
Activation energy directly affects the reaction rate. If the energy barrier is higher (larger Ea), fewer molecules will have sufficient energy to participate in the reaction at a given temperature, leading to a slower reaction rate. Conversely, a lower energy barrier allows more molecules to react, resulting in a faster reaction.
Examples & Analogies
Imagine a group of students (molecules) trying to jump over a fence (activation energy). If the fence is tall (high activation energy), only a few students can jump over it. However, if the fence is shorter (low activation energy), many more students can easily jump over, speeding up the movement across the yard (the reaction).
Key Concepts
-
Activation Energy: The energy barrier required for reactants to form products, crucial for understanding reaction rates.
-
Transition State: The point of highest energy in a reaction pathway, representing the arrangement of atoms at the peak energy.
-
Arrhenius Equation: Mathematical representation relating reaction rate constants to activation energy and temperature, useful for predicting behavior in chemical kinetics.
-
Maxwell-Boltzmann Distribution: Shows the distribution of molecular energies and indicates how temperature affects reaction rates.
Examples & Applications
An example of an endothermic reaction is the thermal decomposition of calcium carbonate (CaCO3), with a high activation energy, requiring heat to initiate decomposition.
Combustion reactions of hydrocarbons like methane (CH4) have lower activation energy due to initial energy supplied by a flame, resulting in rapid reaction rates.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Energy high, reaction’s nigh; low activation, the product’s fly!
Stories
Imagine a race where each runner needs a specific energy boost to leap over a tall barrier; those who can’t reach the top stay behind, just like molecules needing enough energy to react.
Memory Tools
For 'Ea', remember: 'Energy Activation' is All.
Acronyms
Ea - 'Energy needed to activate' for reactions!
Flash Cards
Glossary
- Activation Energy (Ea)
The minimum energy required for reactants to undergo a successful reaction.
- Transition State (E‡)
The highest-energy state of the system during a reaction that occurs at the peak of the energy barrier.
- Arrhenius Equation
An equation that relates the rate constant to activation energy and temperature, expressed as k = A * exp(-Ea/(R*T)).
- MaxwellBoltzmann Distribution
A statistical distribution of molecular speeds in a gas, indicating that only a fraction of molecules will possess sufficient energy to react.
Reference links
Supplementary resources to enhance your learning experience.
