Rate-Determining Step and the Steady-State Approximation
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Rate-Determining Step
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are going to learn about the rate-determining step, which is the slowest step in a multi-step reaction mechanism. Can anyone tell me why knowing the RDS is important?

I think it helps us understand the overall speed of the reaction!

Exactly! The RDS sets the pace for the overall reaction. If we know which step is slow, we can predict how the reaction rate will respond to changes in concentration or temperature.

What happens if the RDS involves an intermediate?

Good question! When the RDS involves intermediates, we often use the steady-state approximation to simplify our analysis.
Understanding Steady-State Approximation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

The steady-state approximation assumes that the concentration of any intermediate remains relatively constant throughout the reaction. Why do you think this is a useful assumption?

It sounds like it helps us avoid dealing with changing concentrations during complex calculations.

Exactly! By assuming the intermediate concentrations are constant, we can focus on the stable reactants and derive a rate law that describes our overall reaction effectively.

Can you give an example of how we would apply this in a reaction?

Sure! If we have a reaction where A reacts to form an intermediate I, which then reacts to form products, we would set up differential equations for the formation and consumption of I and solve under the steady-state assumption.
Applying Rate-Determining Step and Steady-State
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s take a two-step mechanism: A + B forms intermediate I, which then decomposes to products. How would we identify the RDS?

We would look for the slower step, right?

Correct! If we find that the second step is much slower than the first, it will be our RDS. Now, if we apply the steady-state approximation, how would that change our focus?

We can write the rate law in terms of A and B, not I!

Exactly! The key takeaway is to simplify reactions and connect our findings to the overall reaction rate without having to track intermediates.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In multi-step reactions, one step often controls the overall reaction rate, known as the rate-determining step (RDS). The steady-state approximation allows chemists to analyze reaction mechanisms with intermediate species more effectively by assuming their concentration remains constant during the reaction.
Detailed
Rate-Determining Step and the Steady-State Approximation
In chemical kinetics, especially in multi-step reactions, the rate-determining step (RDS) is crucial as it is the slowest step that limits the overall rate of the reaction. This principle helps chemists understand how different steps in a reaction mechanism contribute to the overall speed at which products form. In cases where intermediate species are formed, the steady-state approximation facilitates the derivation of rate laws.
Key Concepts:
- Rate-Determining Step (RDS): The slowest step in a reaction mechanism that determines the rate of the overall reaction.
- Steady-State Approximation: A method used when analyzing mechanisms involving intermediates, which assumes that the concentration of intermediates remains constant throughout the reaction.
Significance:
This is vital for predicting reaction kinetics and validating proposed mechanisms against experimental data.
Youtube Videos

Audio Book
Dive deep into the subject with an immersive audiobook experience.
Rate-Determining Step (RDS)
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In many multi-step mechanisms, one step—the rate-determining step (RDS)—is significantly slower than all the others. This 'bottleneck' step controls the overall reaction rate. If the RDS involves only reactants (no unstable intermediates), its rate law often matches the observed overall rate law directly.
Detailed Explanation
The rate-determining step (RDS) is the slowest step in a series of reactions. Think of a line of cars trying to exit a parking garage: if one car is moving slowly, it holds up the entire line. Similarly, in a reaction mechanism, if one step is much slower than all others, it dictates how quickly the entire reaction proceeds. For instance, if a reaction has several steps, like A turning into an intermediate I and then into products, if forming products from I takes longer than any other step, I becomes the bottleneck, and the rate law for this step can often represent the overall reaction.
Examples & Analogies
Imagine a team of workers trying to finish a project but one worker is stuck waiting for parts to arrive while everyone else is ready to work. No matter how fast they are, they can't progress until he gets his parts. This illustrates how the RDS can dictate the overall speed of a process.
Steady-State Approximation
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If the RDS involves an intermediate species I, the steady-state approximation is invoked: Steady-State Approximation Assume the concentration of any intermediate I remains very small and nearly constant during most of the reaction, so d[I]/dt ≈ 0.
Detailed Explanation
The steady-state approximation is a method used in reaction kinetics when dealing with intermediates—these are species formed in one step and consumed in another. The approximation suggests that the concentration of an intermediate remains constant throughout the reaction, particularly because it is being created and consumed at approximately the same rate. This leads to a simplification where we can treat the intermediate's concentration as constant during the time we analyze the reaction, allowing us to derive rate laws that depend only on reactants.
Examples & Analogies
Think of a bathtub filling with water while simultaneously draining. If the inflow and outflow rates are roughly equal, there won't be a significant change in the water level despite the continuous flow. This is akin to an intermediate in a reaction being created and consumed at constant rates, making its concentration appear stable or 'steady' over time.
Applying the Steady-State Approximation
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Under this assumption, one writes differential rate expressions for the formation and consumption of I, sets d[I]/dt = 0, solves for [I] in terms of the concentrations of stable reactants, and substitutes that into the rate law for the RDS. This yields an overall rate law expressed solely in terms of reactants, with no unknown intermediate concentrations.
Detailed Explanation
When we assume that the concentration of an intermediate remains consistent, we can create equations for how quickly that intermediate is generated and consumed. By setting the rate of change of the intermediate concentration (d[I]/dt) to zero, we can solve for the concentration of the intermediate based on stable reactants. Once we have this relation, we can insert it into the rate law of the rate-determining step, resulting in an overall rate law that describes the entire reaction only in terms of the original reactants, simplifying the kinetic analysis.
Examples & Analogies
Imagine a bakery where dough is constantly being made (formed) and baked (consumed). If the dough made for baking stays roughly the same amount, we can describe the total operations in terms of the amount of flour and sugar used without tracking every individual batch of dough. This simplifies our accounting, much like how the steady-state approximation simplifies rate laws.
Example: Two-Step Mechanism
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
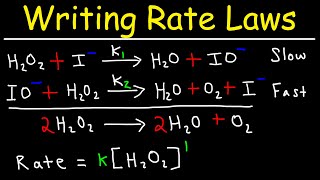
Consider the mechanism: 1. A + B ⇌ I (fast equilibrium, rate constants k₁ forward and k₋₁ reverse) 2. I → products (slow, rate constant k₂) Step 1 establishes an equilibrium with constant K_eq = k₁ / k₋₁ = [I] / ([A]·[B]) so [I] = K_eq · [A] · [B]. Step 2 is the RDS, so Rate = k₂ · [I] = k₂ · (K_eq · [A] · [B]) = (k₂ · K_eq) · [A] · [B]. Hence, the overall rate law is Rate = k_obs · [A] · [B], where k_obs = k₂ · K_eq. This is second order overall (first order in A and first order in B), matching experiment if that is the observed dependence.
Detailed Explanation
In this model, the first step quickly reaches an equilibrium where reactants A and B turn into the intermediate I. Since this step happens fast, we can represent the concentration of I in terms of A and B using an equilibrium constant (K_eq). The second step then takes a longer time, and since it's the rate-determining step, we can derive the rate of the overall reaction based on the steady-state approximation, resulting in a rate law that shows dependency on the concentrations of A and B, confirming observed experimental data. This is essential to ensure that our theoretical model matches real-world reactions.
Examples & Analogies
Think of passengers boarding a train. First, passengers arrive (A and B), quickly filling the station (creating the intermediate I). The train (the slow step) then departs. The rate at which the train leaves depends on how quickly passengers arrive and are processed at the station. We can represent this system in terms of just A and B, simplifying our understanding of how many people can board over time, just like how we derive the rate law focusing only on reactants.
Key Concepts
-
Rate-Determining Step (RDS): The slowest step in a reaction mechanism that determines the rate of the overall reaction.
-
Steady-State Approximation: A method used when analyzing mechanisms involving intermediates, which assumes that the concentration of intermediates remains constant throughout the reaction.
-
Significance:
-
This is vital for predicting reaction kinetics and validating proposed mechanisms against experimental data.
Examples & Applications
In a two-step reaction mechanism where A forms an intermediate I and then I forms products, if I is formed quickly but reacts slowly, the step involving I is the RDS.
The steady-state approximation can apply to enzyme kinetics where the enzyme-substrate complex concentration remains constant.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In reactions where rates compete, the RDS is always the slowest feat.
Stories
Imagine a race with multiple runners, the one who takes the longest determines the pace for all.
Memory Tools
Remember RDS: Really Delays Speed!
Acronyms
RDS
Rate-Determining Step
Flash Cards
Glossary
- RateDetermining Step (RDS)
The slowest step in a multi-step reaction that limits the overall rate.
- SteadyState Approximation
An assumption that the concentration of intermediates remains constant during most of the reaction.
Reference links
Supplementary resources to enhance your learning experience.
