Common Rate Laws: Zero, First, and Second Order
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Zero-Order Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're starting with zero-order reactions. In a zero-order reaction, the rate is constant and independent of the concentration of the reactants. This often occurs when a catalyst's surface is saturated.

How is the rate law for a zero-order reaction defined?

Great question! The rate law is simply Rate = k, where k is the rate constant. Can you tell me what the integrated form looks like?

Is it [A]_t = [A]_0 - kt?

Exactly! And what about the half-life for a zero-order reaction? Does it depend on the initial concentration?

Yes, it depends on [A]_0, right? It’s t₁₋₂ = [A]_0/(2k).

Yes, perfect! If we plot [A] versus time, what do we expect to see?

A straight line with a slope of -k.

Great job! Zero-order reactions are important when the concentration doesn’t affect the rate, typically involving enzyme saturation or catalyst reactions.
First-Order Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's delve into first-order reactions. In a first-order reaction, the rate is directly proportional to the concentration of one reactant: Rate = k[A].

What is the differential form for a first-order reaction?

It is d[A]/dt = -k[A]. Can anyone tell me the integrated form?

It’s ln([A]_t) = ln([A]_0) - kt!

Correct! And the half-life? What’s unique about it?

The half-life is t₁₋₂ = 0.693/k and it’s independent of the initial concentration [A]_0.

Exactly! This means the half-life remains constant regardless of how much reactant you start with. What kind of plot can we use to confirm first-order kinetics?

A plot of ln([A]) versus time, which should yield a straight line.

Well done! First-order reactions are common in processes like radioactive decay.
Second-Order Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's discuss second-order reactions. These can come in two forms: either two molecules of the same reactant or one of two different reactants.

What’s the rate law for two identical reactants?

For two identical reactants, it’s Rate = k[A]^2. And what about for two different reactants?

That would be Rate = k[A][B]!

Excellent! Can anyone share the differential form for a second-order reaction with one reactant?

It’s -d[A]/dt = k[A]^2.

Right! And how do we integrate this for two identical reactants?

The integrated form would be 1/[A]_t = 1/[A]_0 + kt.

Very good! What's the half-life formula for this reaction?

It’s t₁₋₂ = 1/(k[A]_0); notice it depends on the initial concentration.

Exactly! Hence, second-order kinetics reactants will produce a plot of 1/[A] versus time that shows a straight line confirming the reaction order.
Summary of Reaction Orders
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To wrap up today's lesson, can anyone identify the major differences between zero, first, and second-order reactions?

Sure! Zero-order has a constant rate regardless of concentration, first-order's rate changes with concentration, and second-order's rate depends on the square of the concentration.

Correct! What about the half-life in each case?

For zero-order, it depends on the initial concentration; first-order is constant, and for second-order, it also depends on concentration.

Excellent summary! Understanding these differences is critical for predicting reaction behavior in chemical kinetics.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, we explore the rate laws corresponding to different reaction orders: zero, first, and second order. Each order's rate law, reaction characteristics, integrated forms, and half-life expressions are discussed, providing a comprehensive understanding of how reaction rates depend on reactant concentrations.
Detailed
Common Rate Laws: Zero, First, and Second Order
Chemical reactions can be classified by their reaction order, which influences how the rate of reaction changes as reactant concentrations vary. This section specifically details zero, first, and second-order reactions, including their characteristics and mathematical expressions.
Zero-Order Reactions
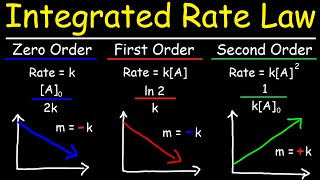
- Rate Law: Rate = k
- Differential Form: d[A]/dt = -k
- Integrated Form: [A]_t = [A]_0 - k·t
- Half-Life (t₁₋₂): t₁₋₂ = [A]_0 / (2k), indicating dependence on the initial concentration [A]_0.
- Graphical Test: A plot of [A] versus time yields a straight line, demonstrating constant behavior, typical when the catalyst surface is saturated.
First-Order Reactions
- Rate Law: Rate = k [A]
- Differential Form: d[A]/dt = -k[A]
- Integrated Form: ln([A]_t) = ln([A]_0) - k·t
- Half-Life (t₁₋₂): t₁₋₂ = 0.693/k, which is independent of [A]_0.
- Graphical Test: A plot of ln([A]) versus time also yields a straight line, characteristic of many unimolecular decompositions.
Second-Order Reactions
- Subtypes include scenarios with two identical reactants or one of two different reactants:
- Rate Law: Rate = k [A]^2
- Rate Law: Rate = k [A][B]
- Differential Form: d[A]/dt = -k[A]^2 in case 1.
- Integrated Form: 1/[A]_t = 1/[A]_0 + kt (for case 1).
- Half-Life (t₁₋₂): t₁₋₂ = 1 / (k[A]_0) for case 1.
- Graphical Test: A plot of 1/[A] versus time results in a straight line.
By understanding these laws and their characteristics, chemists can predict how varying the concentrations of reactants influences the overall rate of a chemical reaction.
Youtube Videos


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Zero-Order Reactions
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Rate law: Rate = k.
● Differential form: d[A]/dt = –k.
● Integrated form: [A]_t = [A]_0 – k·t.
● Half-life t₁₋₂ (time to reduce [A] to half of [A]_0):
t₁₋₂ = [A]_0 / (2 k).
Notice that t₁₋₂ depends on [A]_0.
● Graphical test: Plot [A] versus t; you get a straight line of slope –k.
Zero-order behavior can occur when a catalyst surface is saturated, so increasing [A] no longer increases the rate.
Detailed Explanation
Zero-order reactions have a constant rate that does not depend on the concentration of reactants. In these reactions, the rate is simply equal to the rate constant, k. The differential form of the rate law tells us how the concentration of the reactant A changes over time; it decreases at a steady rate defined by the constant k, which is the slope of the graph when plotting concentration [A] against time t. The integrated form allows us to calculate the concentration of A at any time t, and we see that the half-life also depends on the initial concentration [A]_0. Thus, if we have a zero-order reaction, doubling the initial concentration will double the half-life time.
Examples & Analogies
Imagine a runner on a straight path at a constant speed, which represents a zero-order reaction. No matter how many friends cheer at the sidelines, the runner keeps their pace constant regardless of their enthusiasm (which symbolizes reactant concentration). If the runner takes a drink (which could represent a saturated catalyst), that does not affect their maximum speed; instead, they continue at that steady pace.
First-Order Reactions
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Rate law: Rate = k [A].
● Differential form: d[A]/dt = –k [A].
● Integrated form: ln([A]_t) = ln([A]_0) – k·t.
● Half-life t₁₋₂ is independent of [A]_0:
t₁₋₂ = (ln 2) / k ≈ 0.693 / k.
● Graphical test: Plot ln([A]) versus t; you get a straight line with slope –k.
Many unimolecular decompositions in the gas phase and radioactive decays follow first-order kinetics.
Detailed Explanation
First-order reactions have a rate that is directly proportional to the concentration of one reactant. This means as the concentration of A decreases, the rate of the reaction decreases in a predictable manner. The integrated form of the rate law allows us to convert concentration changes into exponential equations, leading to a straightforward method to find the half-life of the reaction, which is constant at any starting concentration. The graph of ln([A]) versus time yields a straight line, indicating first-order kinetics.
Examples & Analogies
Consider a candle burning. As it burns down (reactant A decreasing), the rate at which wax is consumed (the reaction rate) decreases. The time it takes for the candle to burn halfway (half-life) does not change based on how tall the initial candle was—it's always about the same until it burns out. The more wax left means slower burning, similar to how concentration impacts first-order reactions.
Second-Order Reactions
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Two common scenarios yield second-order kinetics:
1. Two molecules of the same reactant:
2A → products, Rate = k [A]^2.
2. One molecule each of two different reactants:
A + B → products, Rate = k [A][B].
Case 1: Rate = k [A]^2
● Differential form: d[A]/dt = –k [A]^2.
● Integrated form: 1/[A]_t = 1/[A]_0 + k·t.
● Half-life t₁₋₂ = 1 / (k [A]_0).
Note that now t₁₋₂ depends on [A]_0.
● Graphical test: Plot 1/[A] versus t; you get a straight line with slope k.
Case 2: Rate = k [A][B]
If [A]_0 = [B]_0, the integrated form reduces to the same form as Case 1. If [A]_0 ≠ [B]_0, the integrated form is more complicated. In practice, one often simplifies by making one reactant in large excess (pseudo–first-order method; see Section 5.3).
Detailed Explanation
Second-order reactions can occur in two scenarios: either when two molecules of the same reactant collide or when two distinguishable reactants collide. For the first case, the rate increases quadratically with concentration; if we double the concentration of A, the reaction rate quadruples. The integrated form for second-order reactions provides a way to predict concentration changes over time, and the half-life is dependent on the initial concentration of reactants, which differs from first-order kinetics.
Examples & Analogies
Think of a dance party where two people (A and B) must come together to dance (the reaction). If both A and B are needed together to dance, the more friends dancing (increasing concentration) means exponentially more chances for successful dance pairs to form. If one partner leaves early (lower concentration), fewer dance pairs can happen, and this affects how long the dance party lasts—hence the dependence on initial concentration in second-order reactions.
Key Concepts
-
Zero-Order Reaction: The rate is constant and does not depend on the concentration of reactants.
-
First-Order Reaction: The rate depends linearly on the concentration of a single reactant.
-
Second-Order Reaction: The rate depends on the concentration of one reactant squared or the product of two reactants.
Examples & Applications
Zero-order reactions can occur in enzyme-catalyzed processes with a saturated catalyst.
First-order reactions are exemplified by radioactive decay.
Second-order reactions can occur in reactions where two distinct reactants collide, such as A + B → products.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Zero is flat, one runs straight, two's squared or mixed; that's their fate.
Stories
Imagine a race: Zero-time is always a tie, First is a sprint, and Second makes the competitors fly.
Acronyms
Z for Zero, F for First, S for Second.
Flash Cards
Glossary
- ZeroOrder Reaction
A reaction whose rate is independent of the concentration of reactants.
- FirstOrder Reaction
A reaction whose rate is directly proportional to the concentration of one reactant.
- SecondOrder Reaction
A reaction whose rate is dependent on either the square of the concentration of one reactant or the product of the concentrations of two different reactants.
- Rate Law
An expression that relates the reaction rate to the concentrations of reactants.
- HalfLife (t₁₋₂)
The time required for the concentration of a reactant to decrease to half its initial value.
Reference links
Supplementary resources to enhance your learning experience.
