Maxwell–Boltzmann Distribution of Molecular Energies
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Molecular Energies
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're diving into the Maxwell–Boltzmann distribution, a vital concept in understanding how molecular energies vary at a given temperature. Can anyone tell me what affects molecular kinetic energy?

Is it just temperature?

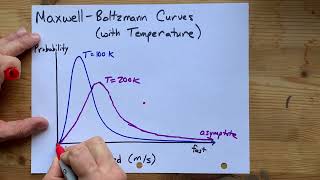
Exactly! Temperature plays a crucial role. The average kinetic energy of gas molecules increases with temperature. Now, the distribution of these energies can be visualized as a curve. Does anyone know how this curve changes with increasing temperature?

I think it broadens and shifts to the right?

Right again! As temperature rises, not only does the curve shift to higher energies, but the spread also widens, which means more molecules can exceed the activation energy for reactions. Let's remember this with the mnemonic: 'Hotter = Higher Energy'!

That’s a good way to remember it!

Great! So when we refer to the fraction of molecules with energy greater than the activation energy, we express this as exp(–Ea/(R·T)). Can anyone explain what each part represents?

Ea is the activation energy, R is the gas constant, and T is temperature?

Exactly! Let's summarize: increased temperature broadens the kinetic energy distribution, increasing the fraction of molecules surpassing the activation energy, which means reaction rates typically increase!
Impact of Temperature on Reaction Rates
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's explore how this energy distribution affects reaction kinetics. How do you think more molecules having the necessary energy impacts reaction rates?

It should increase the reaction rate because more collisions can be effective!

Exactly! More effective collisions mean faster reactions. Remember, reactions only occur when molecule collision energies meet or exceed the activation energy. Why do some reactions fail even if molecules collide frequently?

Because they might not have the right orientation or enough energy?

Spot on! Now let's think about real-life examples. Who can name a scenario where temperature drastically changes reaction rates?

I think about cooking! If you increase the heat, food cooks faster because chemical reactions speed up!

Perfect example! Just like cooking, understanding the Maxwell–Boltzmann distribution helps us predict how reactions will behave with temperature changes!
Practical Implications of Molecular Energies
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's apply what we've learned. How can industries take advantage of the Maxwell–Boltzmann distribution theory in chemistry?

They can optimize reaction conditions, like temperature, to speed up manufacturing processes!

Correct! Industries often tweak conditions based on energy distributions to enhance productivity. Can anyone think of another area where this concept is crucial?

Maybe in climate science when discussing gas behaviors?

Absolutely! The Maxwell–Boltzmann distribution helps us understand atmospheric reactions, pollution behavior, and even reaction kinetics in biology. Let’s summarize: the distribution of molecular energies profoundly impacts not only industrial processes but also environmental and biological systems. Who can recall why this is important?

Improving efficiency and reducing harmful impacts!

Yes! Understanding molecular energies helps make scientists and engineers more responsive to our planet's needs. Excellent discussion today, everyone!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The Maxwell–Boltzmann distribution illustrates that at any given temperature, the kinetic energies of gas molecules vary. As temperature increases, more molecules exceed activation energy, affecting reaction rates. This concept underpins the relationship between molecular energy distributions and chemical kinetics.
Detailed
Maxwell–Boltzmann Distribution of Molecular Energies
The Maxwell–Boltzmann distribution is a statistical representation of the distribution of kinetic energies among molecules in a gas. Key points include:
- Temperature Effect: At a constant temperature T, molecules possess a range of kinetic energies, characterized by the distribution curve. As temperature increases, the curve broadens and shifts, meaning a larger fraction of molecules will have energies equal to or greater than the activation energy (Ea).
- Activation Energy: The fraction of molecules with sufficient kinetic energy to overcome the activation energy barrier is approximately given by the expression: exp(–Ea/(R·T)). This exponential relationship highlights the importance of molecular energy in chemical reactions.
- This distribution is crucial for understanding reaction kinetics, as it links the concept of energy distribution with the frequency of effective collisions among reactants. Thus, it provides a molecular-level insight into why increasing temperature can result in faster reaction rates by increasing the number of molecules that can participate in effective collisions.
Youtube Videos


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Overview of the Maxwell–Boltzmann Distribution
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
At any given temperature T, the kinetic energies of molecules in a gas follow the Maxwell–Boltzmann distribution. The key points are:
- As T increases, the distribution broadens and shifts toward higher energies, so more molecules have energy above any fixed threshold (such as the activation energy).
- The fraction of molecules with kinetic energy greater than or equal to Ea is approximately exp(–Ea/(R·T)).
Detailed Explanation
The Maxwell–Boltzmann distribution describes how the speeds and kinetic energies of molecules in a gas vary at a given temperature. As the temperature increases, the average kinetic energy of the molecules also increases, resulting in a wider range of energies. Consequently, at higher temperatures, more molecules possess enough energy to overcome the energy barrier required for a reaction (known as activation energy, Ea).
In mathematical terms, the fraction of molecules with kinetic energy equal to or greater than the activation energy is characterized by the equation exp(–Ea/(R·T)), where R is the universal gas constant and T is the temperature in Kelvin. This exponential relationship illustrates that even a small increase in temperature can dramatically increase the number of molecules that can effectively participate in a reaction.
Examples & Analogies
Imagine a group of runners competing in a race. At a lower temperature (or when the competition is less intense), only a few athletes can sprint fast enough to reach the finish line in record time, representing the molecules that have enough energy to react. However, as the heat (or competition) increases, more and more runners can push themselves to go faster and cross the finish line. Similarly, as temperature rises, more molecules in a gas achieve the necessary kinetic energy to react, leading to increased reaction rates.
The Arrhenius Expression
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When multiplied by the collision frequency Z_AB and by the steric factor p, this exponential term leads directly to the Arrhenius expression for the rate constant (see Section 3.5).
Detailed Explanation
The Arrhenius expression relates the rate constant of a reaction, k, to its activation energy and temperature. According to the collision theory, for a reaction to occur, molecules must collide with sufficient energy and in the correct orientation. The term exp(–Ea/(R·T)) accounts for the fraction of molecules that successfully achieve this requirement, based on their kinetic energies at a given temperature.
The rate constant, k, incorporates both the collision frequency (how often molecules collide) and the steric factor (the fraction of collisions that are effective). Essentially, this relationship allows us to quantitatively understand how changes in temperature affect reaction rates by showing that higher temperatures increase k, thus speeding up the reaction.
Examples & Analogies
Think of the Arrhenius equation like a light switch. When it's cold (lower temperatures), only a few light bulbs (molecules) turn on (react), representing minimal activity. However, as you increase the temperature (like turning up the heat in a room), more bulbs flicker to life, symbolizing that more molecules can reach the activation energy needed to react. This analogy simplifies understanding how temperature influences molecular behavior and reaction kinetics.
Key Concepts
-
Maxwell-Boltzmann Distribution: Describes how molecular energies in a gas are distributed at a given temperature.
-
Activation Energy: The minimum energy that reacting species must possess for a reaction to occur.
-
Temperature Effect: Increasing temperature broadens the distribution, resulting in more molecules capable of overcoming the activation energy barrier.
Examples & Applications
In an increase from 300 K to 310 K, the number of molecules with sufficient energy to overcome the activation energy doubles, accelerating a reaction.
Cooking food at a higher temperature facilitates faster chemical reactions, enhancing flavors and textures.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When the heat's okay, molecules play, gaining energy every day!
Stories
Imagine cooking pasta; as the water heat increases, not only do the noodles soften, but so do the chemical bonds in the flavors, enhancing taste as energy rises!
Memory Tools
TEMPERATURE increases means MORE energy molecules exceed activation (TMME).
Acronyms
KERM
Kinetic Energy
Energy Barrier (Activation)
Reaction Rates
Maxwell–Boltzmann Distribution.
Flash Cards
Glossary
- Maxwell–Boltzmann Distribution
A statistical representation of the distribution of molecular energies in a gas at a given temperature.
- Activation Energy (Ea)
The minimum energy barrier that reacting molecules must overcome for a reaction to occur.
- Kinetic Energy
The energy that a molecule possesses due to its motion.
- Steric Factor (p)
The fraction of collisions that occur with the correct orientation to lead to a chemical reaction.
Reference links
Supplementary resources to enhance your learning experience.
