Buffer Solutions: Resisting pH Change
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Buffer Solutions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we'll explore buffer solutions, which are designed to resist pH changes. Can anyone tell me what a buffer solution is?

Isn't a buffer something that keeps pH stable?

Exactly! A buffer solution can resist significant pH changes when small amounts of acids or bases are added. So, why do you think maintaining pH is crucial in chemical processes?

Maybe because many reactions need specific pH levels to function correctly?

Correct. For example, our blood maintains a pH around 7.4! Let’s dive deeper into how buffer solutions work.
Components of Buffer Solutions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Buffer solutions typically contain a weak acid and its conjugate base, or a weak base and its conjugate acid. Can someone give me an example of each?

For a weak acid, isn’t acetic acid a good example paired with sodium acetate?

And for a weak base, ammonia goes with ammonium chloride!

Well done! Now, let's discuss how these components interact to stabilize pH. When you add an acid, how do our buffer components respond?

The conjugate base neutralizes the added H⁺ ions!

Right! This helps prevent a sharp decrease in pH. Great connection!
Mechanisms of Buffer Action
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s look deeper into how buffers function. Suppose we add a small amount of acid to our buffer. What reaction occurs?

The H⁺ ions will react with the conjugate base to form the weak acid.

Exactly! It minimizes the change in H⁺ concentration. Now, what if we added a base?

The weak acid would react with the OH⁻ to form water and the conjugate base!

Spot on! This interaction is critical for resisting changes in pH. Now, what factors influence buffer capacity?

I think it has to do with the concentrations of the buffer components?

Correct! Higher concentrations provide greater capacity, and having equal concentrations is particularly effective.
Henderson-Hasselbalch Equation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

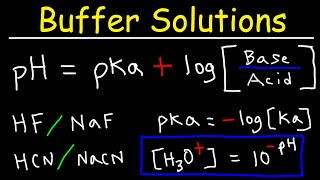
Now, let’s look at the Henderson-Hasselbalch equation, which helps us calculate the pH of buffer solutions. Can anyone state what it is?

'pH = pKa + log10([A−]/[HA])'?

Exactly! This equation shows us the relationship between the pH, the acid dissociation constant, and the concentrations of the acid and its conjugate base. What happens when [A−] equals [HA]?

The pH then equals pKa!

Exactly! Buffers work best when their pH is near their pKa. Excellent connection!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
A buffer solution maintains a stable pH by utilizing a weak acid and its conjugate base or a weak base and its conjugate acid to neutralize added acids or bases. The concept is key in various biological and chemical processes, emphasizing buffer capacity and the Henderson-Hasselbalch equation for practical application.
Detailed
Detailed Summary
Buffer solutions play a vital role in maintaining a stable pH level in chemical and biological systems. These solutions consist of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid, which work in equilibrium to neutralize small amounts of added acid or base.
Composition of Buffer Solutions
- Acidic Buffer: Combines a weak acid (e.g., Ethanoic acid, CH₃COOH) with its conjugate base (e.g., Sodium ethanoate, CH₃COONa).
- Basic Buffer: Coordinates a weak base (e.g., Ammonia, NH₃) with its conjugate acid (e.g., Ammonium chloride, NH₄Cl).
Buffer Mechanism
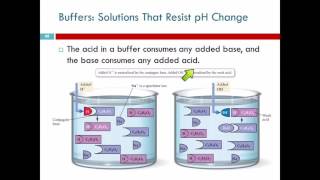
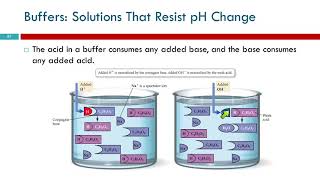
- Adding Acid (H⁺): The added H⁺ is neutralized by the conjugate base, pulling the equilibrium leftward and controlling the increase in [H⁺].
- Adding Base (OH⁻): The conjugate acid consumes the added OH⁻, minimizing the rise in [OH⁻] and thus stabilizing the pH.
Buffer Capacity
The effectiveness of a buffer, known as buffer capacity, hinges on the concentrations of its components and the ratio of the weak acid to its conjugate base, ideally close to equal concentration for maximum efficacy.
Henderson-Hasselbalch Equation
This equation, pH = pKa + log10([A−]/[HA]), is essential for calculating the pH of buffer solutions, particularly indicating that the buffer works best at pH values close to its pKa.
Youtube Videos
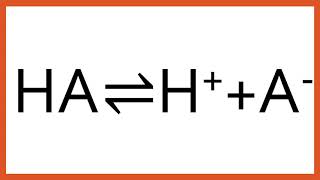




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Buffer Solutions
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A buffer solution is a remarkable chemical system that exhibits resistance to significant changes in pH upon the addition of small amounts of acid or base. This ability to maintain a relatively stable pH is indispensable in countless chemical and biological processes, such as regulating the pH of blood plasma or in pharmaceutical formulations.
Detailed Explanation
A buffer solution can resist changes in pH when small amounts of acids or bases are added. This means that even if you add something acidic or basic, the pH of the buffer doesn't change significantly. This is crucial in living systems, like our blood, which needs to maintain a very specific pH for proper functioning. An example of this is the use of buffered solutions in medical settings where maintaining a stable pH is vital for patient health.
Examples & Analogies
Imagine a sponge that can soak up small amounts of water without getting saturated. Similarly, a buffer is like a sponge for hydrogen ions (H⁺) or hydroxide ions (OH⁻); it can absorb these ions without allowing big changes in pH.
Composition of Buffer Solutions
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A buffer solution is composed of a specific mixture of components:
1. A weak acid and its conjugate base:
- Example: Ethanoic acid (CH3 COOH) and sodium ethanoate (CH3 COONa).
The ethanoate ion (CH3 COO−) is the conjugate base of ethanoic acid.
2. A weak base and its conjugate acid:
- Example: Ammonia (NH3) and ammonium chloride (NH4Cl). The ammonium ion (NH4+) is the conjugate acid of ammonia.
Detailed Explanation
Buffers can be made up of two different compositions: The first type includes a weak acid and its conjugate base, like ethanoic acid paired with sodium ethanoate. The second type consists of a weak base and its conjugate acid, such as ammonia and ammonium chloride. This combination is important because the weak acid/base can react with any strong acid or base that might disrupt the pH balance, helping to maintain stability.
Examples & Analogies
Think of a buffer as a team consisting of two players. One player (the weak acid) can take in the extra challenges (H⁺ ions) when the other player (the conjugate base) isn't able to handle them alone. Together, they work dynamically to respond to changes and keep the pH in check, just like a sports team that adapts to different opponents during a game.
How Buffers Work
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
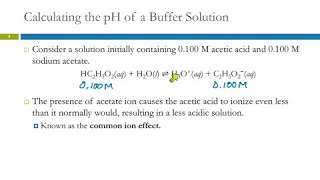
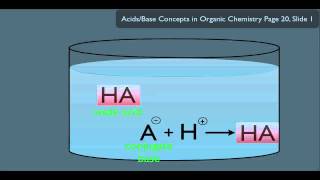
The key to a buffer's action lies in the presence of both the weak acid/base and its corresponding conjugate, which exist in equilibrium. Let's consider an acidic buffer composed of a weak acid HA and its conjugate base A−:
HA(aq)⇌H+(aq)+A−(aq)
● When a small amount of acid (H⁺) is added:
The added H+ ions are consumed by reacting with the relatively high concentration of the conjugate base (A−) already present in the buffer solution. H+(aq)+A−(aq)→HA(aq)
● When a small amount of base (OH−) is added:
The added OH− ions are consumed by reacting with the relatively high concentration of the weak acid (HA) present in the buffer solution. HA(aq)+OH−(aq)→A−(aq)+H2 O(l)
Detailed Explanation
Buffers work through a balance between the weak acid and its conjugate base. In the case of an acidic buffer, when you add more acid (H⁺ ions), the conjugate base will react with those ions to minimize pH changes. Conversely, if you add a strong base (OH⁻ ions), the weak acid will react with the base to prevent the pH from rising too much. This dual action is what allows buffers to maintain a stable pH.
Examples & Analogies
Imagine you're building a sandcastle at the beach. If waves (representing added acids or bases) try to wash away your castle, you have buckets and shovels (representing the weak acid and conjugate base) ready. If the waves get stronger, you quickly rebuild with what you have to keep the castle standing. Just like that, buffers adapt to maintain the desired pH.
Buffer Capacity
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Buffer capacity refers to the amount of acid or base that a buffer solution can neutralize before its pH begins to change significantly. The capacity of a buffer depends on two primary factors:
● Concentrations of the Buffer Components: Higher concentrations of both the weak acid/base and its conjugate provide a greater buffer capacity, as there are more species available to react with added acid or base.
● Ratio of Components: Buffers are most effective when the concentrations of the weak acid and its conjugate base (or weak base and its conjugate acid) are approximately equal (i.e., [HA]≈[A−]). At this point, the buffer can neutralize roughly equal amounts of added acid or base.
Detailed Explanation
The effectiveness of a buffer solution is referred to as its buffer capacity. This depends on the concentration of the weak acid and its conjugate base. If you have a lot of both components, the buffer can handle more added acid or base before the pH changes. Moreover, a buffer works best when these two components are in similar amounts, since this balance allows the buffer to respond equally to either acids or bases that are added.
Examples & Analogies
Imagine a person's ability to fight off an infection. If they have a strong immune system (high buffer capacity) with plenty of resources (like immune cells) ready to fight, they can handle a larger number of pathogens (added acids or bases) without becoming sick (changing pH). If their immune system is not as strong or balanced, even a small infection might make them ill.
Henderson-Hasselbalch Equation
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For an acidic buffer, the Ka expression can be rearranged to derive the Henderson-Hasselbalch equation, a very useful tool for calculating the pH of a buffer solution:
pH=pKa +log10 ([weak acid][conjugate base])
For the general case of HA⇌H++A−:
pH=pKa +log10 ([HA][A−])
This equation clearly shows that when [A−]=[HA], the log10(1) term becomes 0, and therefore pH=pKa. This confirms that a buffer is most effective at a pH close to its pKa value.
Detailed Explanation
The Henderson-Hasselbalch equation is a formula used to calculate the pH of a buffer solution. It relates the pH to the ratio of the concentration of the weak acid and its conjugate base. This equation helps predict how a buffer will behave under different conditions. When the concentrations of the acid and conjugate base are equal, the pH will equal the pKa, which means the buffer is most effective when pH is around this value.
Examples & Analogies
Think of this equation like a recipe for a perfect smoothie, where the right balance of fruits and liquid gives you the best flavor (pH). If you have equal amounts of both ingredients (like the weak acid and conjugate base), you achieve the best taste (buffer effectiveness). Adjusting these amounts changes the final flavor, just as it would change the pH.
Key Concepts
-
Buffer Solutions: Mixtures that resist pH changes.
-
Weak Acids and Bases: Key components in buffer systems.
-
Conjugate Bases: Formed when weak acids donate protons.
-
Buffer Capacity: Determines the effectiveness of a buffer.
-
Henderson-Hasselbalch Equation: A formula to calculate buffer pH.
Examples & Applications
An ethanoic acid-sodium ethanoate buffer solution neutralizes H+ ions upon acid addition.
An ammonia-ammonium chloride buffer consumes OH- ions to stabilize pH when a base is added.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Buffers hold their ground, as acids and bases surround. They keep pH stable, without making a fable.
Stories
Imagine a balance beam where the acid and base trade places without disturbing the equilibrium, illustrating how buffers maintain stability.
Memory Tools
For acids: 'Weak steals from a strong'; for bases: 'Weak hugs the strong'—highlighting how they interact to stabilize pH.
Acronyms
BASIC
Buffer Against Sudden Input Changes - helping you recall the purpose of buffers.
Flash Cards
Glossary
- Buffer Solution
A solution that resists significant changes in pH when small amounts of acid or base are added.
- Weak Acid
An acid that only partially dissociates in solution.
- Conjugate Base
The species that remains after an acid donates a proton.
- Buffer Capacity
The amount of acid or base that a buffer can neutralize without a significant change in pH.
- HendersonHasselbalch Equation
An equation that relates pH, pKa, and the concentrations of an acid and its conjugate base.
Reference links
Supplementary resources to enhance your learning experience.
