Strength of Acids and Bases: Strong vs. Weak
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Strong Acids
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, let's dive into the concept of strong acids. Can anyone tell me what we mean by a strong acid?

I think a strong acid is one that can donate protons easily?

That's close! A strong acid completely dissociates in water, meaning nearly all of its molecules release their protons. For example, hydrochloric acid (HCl) shows almost total dissociation.

So what does the equilibrium look like for strong acids?

Excellent question! For strong acids, the equilibrium lies almost entirely to the right, favoring the products. Remember, we can use the acronym 'DART'—Dissociate Almost Right for Total dissociation.

Are there examples of strong acids?

Yes, common examples include hydrochloric acid, sulfuric acid, and nitric acid. These are often used in laboratory settings due to their complete ionization.

So for a strong acid like HCl, if I have a 0.1 M solution, the [H⁺] should also be about 0.1 M, right?

Exactly! You’re catching on! The concentration of hydrogen ions will indeed equal the initial concentration of the strong acid.

To summarize, strong acids completely dissociate in solution, and their equilibrium is essentially shifted to the right, leading to high [H⁺] concentrations equal to the acid's initial concentration.
Exploring Weak Acids
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's explore weak acids. What do you think is the key difference between weak and strong acids?

I guess weak acids don't completely dissociate?

Correct! Weak acids partially dissociate in solution. This means that only a small fraction of their molecules donate protons to water.

So, what does the equilibrium look like for weak acids?

For weak acids, the equilibrium position lies predominantly to the left, favoring the reactants. The acronym 'PARTIAL'—Partially Ionized Reactants Together in A Left Lean serves to help you remember this!

Can you give me an example of a weak acid?

Certainly! Ethanoic acid, or acetic acid, is a common weak acid. In a 0.1 M solution, you’ll find that the [H⁺] concentration is much lower than 0.1 M due to partial dissociation.

What do we use to measure their strength then?

We use the acid dissociation constant, Ka, to quantify the strength of a weak acid. A smaller Ka indicates a weaker acid. For practical use, the pKa, which is the negative logarithm of Ka, can be easier to work with.

To summarize, weak acids partially dissociate in aqueous solution, leading to equilibrium positions favoring the reactants, and we measure their strength using the Ka or pKa values.
Dissociation of Strong and Weak Bases
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's switch gears and talk about bases. Can anyone explain the difference between a strong base and a weak base?

I think strong bases completely dissociate while weak bases don't?

Exactly! Strong bases such as sodium hydroxide (NaOH) fully dissociate in solution, producing hydroxide ions. Their equilibrium lies entirely on the product side.

What about weak bases though?

Weak bases partially dissociate or react with water to produce hydroxide ions. An example is ammonia (NH₃). Its expression shows that [OH⁻] concentrations registered are much lower than the initial base concentration!

So how do we measure their strength?

Just as with acids, we employ the base dissociation constant, Kb. A smaller Kb indicates weaker basicity. Similarly, we can use pKb to simplify this measure.

To summarize, strong bases fully dissociate, while weak bases only partially dissociate, and we utilize Kb or pKb values to quantify their strength.
Relationships Between Acid and Base Constants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's discuss the relationship between the dissociation constants for conjugate pairs. Does anyone know what Ka and Kb tell us?

Are they related somehow?

Absolutely! For any conjugate acid-base pair, the product of Ka for the weak acid and Kb for its conjugate base equals Kw, the ion product of water: Ka × Kb = Kw.

How does that help us understand strength?

If you know the strength of an acid from Ka, you can infer that its conjugate base will be weak, and vice versa. This shows a stunning connection in acid-base chemistry!

What’s the stored value of Kw?

Good question! At room temperature, Kw is 1.0 × 10⁻¹⁴. Thus, high Ka suggests low Kb. A quick way of remembering this is to think 'Strong acid weak base, weak acid strong base,' showing the opposites via their dissociation constants.

To summarize, understanding the relationship between Ka, Kb, and Kw enables you to predict the strength of conjugate acids and bases, reinforcing the interconnected nature of these concepts.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
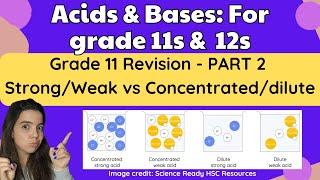
In this section, we describe strong acids as those that completely dissociate in solution, while weak acids only partially dissociate. We explore examples, equilibrium, and the relevant constants, Ka and Kb, alongside relationships between conjugate pairs.
Detailed
Strength of Acids and Bases: Strong vs. Weak
In this section, we elucidate the critical differences between strong and weak acids and bases based on their ionization in aqueous solutions.
Strong Acids
- Definition: Strong acids completely dissociate in solution, meaning almost all acid molecules release their protons into the solution.
- Equilibrium Position: The equilibrium for strong acids lies heavily toward the products, indicating complete ionization.
- Common Examples: Hydrochloric acid (HCl), sulfuric acid (H₂SO₄), and nitric acid (HNO₃) are quintessential strong acids.
- Example Dissociation: HCl in a 0.1 M solution demonstrates that [H⁺] approximates the acid concentration, validating its definition as a strong acid.
Weak Acids
- Definition: Weak acids partially dissociate, resulting in a modest quantity of proton donation.
- Equilibrium Position: The equilibrium lies predominantly to the left, favoring reactants due to incomplete ionization.
- Common Examples: Ethanoic acid (CH₃COOH), carbonic acid (H₂CO₃), and phosphoric acid (H₃PO₄) serve as classic weak acids.
- Example Dissociation: In a 0.1 M solution of CH₃COOH, [H⁺] is significantly less than 0.1 M due to limited dissociation.
- Acid Dissociation Constant (Ka): Defines the strength of a weak acid, with a smaller Ka indicating weaker acidity.
- pKa Value: The pKa offers a convenient measure, where larger values signify weaker acids.
Strong and Weak Bases
- Strong Bases: Analogous to strong acids, these completely dissociate in solution, producing hydroxide ions (e.g., NaOH). They exhibit a similar equilibrium position.
- Weak Bases: Like weak acids, only a fraction of a weak base dissociates, resulting in significantly lower [OH⁻] compared to the initial concentration (e.g., NH₃).
- Base Dissociation Constant (Kb): Measures the strength of weak bases, with smaller Kb signifying weaker basicity.
- pKb Value: Higher pKb values correlate with weaker bases.
Relationship Between Ka, Kb, and Kw for Conjugate Pairs
- The interaction between the acid dissociation constant (Ka) of a weak acid and its conjugate base's dissociation constant (Kb) can be expressed as:
Ka × Kb = Kw,
where Kw is the ion product constant for water (1.0 x 10⁻¹⁴ at 25 °C).
- These relationships illustrate that strong acids have weak conjugate bases, while weak acids possess correspondingly stronger conjugate bases.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Strength of Acids
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Strong Acids
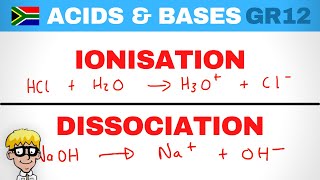
- Definition: Acids that completely dissociate/ionize in an aqueous solution. This means that virtually all the acid molecules donate their protons to water.
- Equilibrium Position: The dissociation reaction lies almost entirely to the right (towards products).
- Common Examples:
- Hydrochloric acid (HCl)
- Sulfuric acid (H2 SO4 )
- Nitric acid (HNO3 )
- Example Dissociation: For 0.1 M HCl: HCl(aq)→H+(aq)+Cl−(aq) In this case, the concentration of hydrogen ions, [H+], will be approximately equal to the initial concentration of the strong acid (e.g., 0.1 M).
Detailed Explanation
Strong acids are defined as those that completely dissociate in water, meaning they release all of their available protons (H+) into the solution. This leads to a high concentration of hydrogen ions, resulting in a low pH. For example, when hydrochloric acid (HCl) is added to water, almost all molecules of HCl turn into H+ and Cl− ions, creating a strong acidic solution. This dissociation is represented by the equilibrium position lying significantly to the right, indicating a predominance of products.
Examples & Analogies
Think of strong acids like a fully opened faucet. When you turn the faucet on all the way, water flows out continuously without holding back. Similarly, strong acids flow freely in terms of releasing protons when dissolved in water.
Strength of Weak Acids
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Weak Acids
- Definition: Acids that partially dissociate/ionize in an aqueous solution. Only a small fraction of the acid molecules donate their protons.
- Equilibrium Position: The dissociation establishes an equilibrium that lies predominantly to the left (towards reactants).
- Common Examples:
- Ethanoic acid (acetic acid, CH3 COOH)
- Carbonic acid (H2 CO3 )
- Phosphoric acid (H3 PO4 )
- Example Dissociation: For 0.1 M CH3 COOH:
CH3 COOH(aq) ⇌ H+(aq) + CH3 COO−(aq) Here, [H+] will be significantly less than 0.1 M.
- Acid Dissociation Constant (Kₐ): For a general weak acid, HA, the equilibrium constant for its dissociation is: Kₐ =[HA][H+][A−]. A smaller Kₐ value indicates a weaker acid (less dissociation).
- pKₐ Value: This is a more convenient way to express acid strength, analogous to pH: pKₐ = −log₁₀(Kₐ). A larger pKₐ value indicates a weaker acid.
Detailed Explanation
Weak acids are those that do not fully dissociate in solution. This means that only a small fraction of the acid's molecules give away protons when dissolved in water, leading to a much lower concentration of hydrogen ions compared to strong acids. For instance, when acetic acid (CH3 COOH) is mixed with water, it only partially breaks down into H+ ions and acetate ions (CH3 COO−), establishing a balance that favors the reactants. The extent of this dissociation is quantified by the acid dissociation constant (Kₐ), where a smaller Kₐ indicates a weaker acid.
Examples & Analogies
Imagine weak acids as a partially opened valve on a hose. The valve allows some water to flow, but much remains inside the hose. Similarly, a weak acid only releases some of its protons into the solution, leaving many intact.
Strength of Bases
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Strong Bases
- Definition: Bases that completely dissociate/ionize in an aqueous solution, producing hydroxide (OH−) ions.
- Equilibrium Position: The dissociation essentially goes to completion, favoring products.
- Common Examples:
- Group 1 hydroxides (e.g., sodium hydroxide (NaOH), potassium hydroxide (KOH))
- Some Group 2 hydroxides (e.g., barium hydroxide (Ba(OH)2 ))
- Example Dissociation: For 0.1 M NaOH: NaOH(aq)→Na+(aq)+OH−(aq) The concentration of hydroxide ions, [OH−], will be approximately equal to the initial concentration of the strong base (e.g., 0.1 M).
Detailed Explanation
Strong bases are defined as substances that completely dissociate in water, resulting in the release of hydroxide ions (OH−) into the solution. For example, sodium hydroxide (NaOH) dissolves completely to yield Na+ and OH− ions, which makes the solution very alkaline. The equilibrium position for this reaction essentially lies to the right, demonstrating that the formation of products is favored.
Examples & Analogies
Think of strong bases like a fire hose that is fully opened. When activated, it releases a powerful flow of water (or hydroxide ions in this case) without holding back, resulting in a highly alkaline solution.
Strength of Weak Bases
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Weak Bases
- Definition: Bases that partially dissociate/ionize (or ionize by accepting a proton from water) in an aqueous solution, producing OH− ions.
- Equilibrium Position: The equilibrium lies predominantly to the left (towards reactants).
- Common Examples:
- Ammonia (NH3 )
- Organic amines (e.g., methylamine, CH3 NH2 )
- Example Dissociation: For 0.1 M NH3 : NH3 (aq) + H2 O(l) ⇌ NH4+ (aq) + OH−(aq) Here, [OH−] will be significantly less than 0.1 M.
- Base Dissociation Constant (Kₐ): For a general weak base, B, the equilibrium constant for its reaction with water is: Kb =[B][BH+][OH−]. A smaller Kb value indicates a weaker base.
- pKₐ Value: pKₐ = −log₁₀(Kb). A larger pKₐ value indicates a weaker base.
Detailed Explanation
Weak bases only partially dissociate in solution, generating hydroxide ions and establishing an equilibrium where the majority of the base molecules remain unchanged. For instance, ammonia (NH3) can accept a proton from water and partially form ammonium ions (NH4+) and hydroxide ions, but not all NH3 molecules will react. The extent of this reaction is described using the base dissociation constant (Kₐ), where a smaller Kₐ indicates a weaker base.
Examples & Analogies
Think of a weak base like a partially opened door. It allows some airflow (or protons in this case), but not as much as a completely open door would. This illustrates how weak bases do not completely react, leading to fewer hydroxide ions in the solution.
Relationship between Acid and Base Strength
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Relationship between Kₐ, Kb, and Kw for Conjugate Pairs
For any conjugate acid-base pair (e.g., a weak acid HA and its conjugate base A−), their dissociation constants are inversely related through the ion product of water (Kₜ):
Kₐ (HA) × Kb (A−) = Kw
Taking the negative logarithm of both sides provides a useful relationship in terms of pK values:
pKₐ (HA) + pKₑ (A−) = pKₜ
At the standard temperature of 25 °C, Kₜ = 1.0 × 10−14, so pKₜ = 14.00. Therefore, for a conjugate pair at 25 °C:
pKₐ + pKₑ = 14.00
This relationship highlights that a strong acid will have a very weak conjugate base, and conversely, a weak acid will have a relatively strong conjugate base.
Detailed Explanation
The relationship between the dissociation constants of a conjugate acid-base pair is crucial in understanding acid-base strength. For any weak acid and its conjugate base, their dissociation constants multiply to equal the ion product of water (Kₜ). When we convert this relationship to pK values, it reveals that the sum of the pKₐ of the weak acid and the pKₑ of its conjugate base always equals the pKₜ (14 at 25 °C). This means that a strong acid has a weak conjugate base (one that hardly dissociates) and vice versa.
Examples & Analogies
You can think of this relationship like a seesaw. When one side (the strong acid) is heavy (has a low pKₐ), then the other side (the weak conjugate base) must be light (has a high pKₑ) to keep the seesaw balanced. This illustrates how the strengths of acids and bases are interconnected.
Key Concepts
-
Strong Acids: Completely dissociate in solution, e.g., HCl.
-
Weak Acids: Partially dissociate in solution, e.g., CH₃COOH.
-
Strong Bases: Completely dissociate to produce OH⁻, e.g., NaOH.
-
Weak Bases: Partially dissociate or react to form OH⁻, e.g., NH₃.
-
Dissociation Constant (Ka): Quantifies the strength of a weak acid.
-
Dissociation Constant (Kb): Quantifies the strength of a weak base.
-
Relationship: Ka × Kb = Kw, illustrating the connection between acid and base strengths.
Examples & Applications
Hydrochloric acid (HCl) is a strong acid that completely ionizes in solution.
Acetic acid (CH₃COOH) is a weak acid that only partially ionizes.
Sodium hydroxide (NaOH) is a strong base that completely dissociates in water.
Ammonia (NH₃) is a weak base that partially reacts with water to form hydroxide ions.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Strong acid, do not fear, completely dissociates, it's crystal clear!
Stories
Imagine a party where a strong acid invites all its friends (protons) and they all show up. In contrast, a weak acid awkwardly invites friends but only a few come.
Memory Tools
Remember 'DART' for strong acids—Dissociate Almost Right for Total.
Acronyms
For weak acids, use 'PARTIAL'—Partially Ionized Reactants Together in A Left Lean.
Flash Cards
Glossary
- Strong Acid
An acid that completely dissociates in an aqueous solution.
- Weak Acid
An acid that partially dissociates in an aqueous solution.
- Dissociation Constant (Ka)
The equilibrium constant for the dissociation of a weak acid, indicating its strength.
- Dissociation Constant (Kb)
The equilibrium constant for the dissociation of a weak base, indicating its strength.
- Equilibrium Position
The position at which the concentration of reactants and products no longer changes over time.
- Conjugate AcidBase Pair
A pair of species that differ by a single proton; one is an acid and the other is its corresponding base.
Reference links
Supplementary resources to enhance your learning experience.
