Quantitative Measures: pH, pOH, and K_w
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Ion Product of Water (K_w)
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we'll discuss the ion product of water, denoted as K_w. This reflects the equilibrium established during the autoionization of water. Can anyone tell me what that means?

Does it mean that water can form hydrogen ions and hydroxide ions?

Exactly! The equation is H₂O ⇌ H⁺ + OH⁻. The constant associated with this reaction, called K_w, is 1.0 × 10⁻¹⁴ at 25°C. What can you infer about the concentrations of these ions in pure water?

They must be equal since water is neutral!

Correct! In neutral water at 25°C, both [H⁺] and [OH⁻] are 1.0 × 10⁻⁷ M. Remember, in acidic solutions, [H⁺] is greater than [OH⁻], and vice versa for basic solutions. Let's remember this relationship using the acronym K_a-Always.

How does temperature affect K_w?

Great question! K_w is temperature-dependent. As temperature increases, K_w also increases. Let's keep that in mind as we further explore pH and pOH.
Understanding pH
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand K_w, let’s dive into pH. The pH scale is logarithmic, expressed as -log₁₀[H⁺]. What do you think that means for measuring acidity?

Does it mean small changes in [H⁺] lead to large changes in pH?

Exactly! Each unit change in pH represents a tenfold change in hydrogen ion concentration. For instance, a solution with a pH of 3 has ten times the [H⁺] of a solution with a pH of 4. Can anyone identify the pH of pure water?

That should be 7!

Yes, right! Remember that a pH less than 7 indicates acidic conditions while greater than 7 indicates basic conditions. What could be a practical example where this matters?

In biological systems? Like how our blood needs to maintain a pH around 7.4?

Perfect example! pH control is vital in many chemical and biological systems. Let's recap: pH measures hydrogen ion concentration, influencing acidity or alkanity. Keep practicing the pH formula to solidify your understanding!
Exploring pOH
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss the pOH scale. Like pH, it quantifies the hydroxide ion concentration as pOH = -log₁₀[OH⁻]. How do you think it relates to pH?

If pH increases, then wouldn't pOH decrease?

Exactly! The relationship pH + pOH = 14 holds true at 25°C. If you know one, you can easily find the other. Could you give me an example of calculating pH if I told you [OH⁻] = 1.0 × 10⁻⁴ M?

First, I would find pOH, which is 4, and then do 14 - 4 to get a pH of 10.

Spot on! That's how we can quickly assess solution properties. Now, keep that in mind when working with strong and weak acids. Let’s wrap it up: pOH is the hydroxide counterpart of pH and is critical in determining solution behavior.
Calculating pH for Strong and Weak Acids
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's apply what we learned! How would you find the pH of a strong acid, say HCl with a concentration of 0.1 M?

Because HCl is a strong acid, I can directly use its concentration, so pH = -log₁₀[0.1], which equals 1.

Excellent! What about a weak acid like acetic acid at the same concentration?

For weak acids, I need to find Ka and set up an ICE table because it doesn’t fully dissociate.

Exactly! You set up your equilibrium, solve for x, and then find [H⁺] to calculate pH. It’s a bit more complex. Would anyone like to practice this?

Sure, a practical example would be great!

Okay! If we have 0.1 M acetic acid with Ka = 1.8 × 10⁻⁵, set up the problem, and let’s solve it together. Recap: strong acids use concentration directly, while weak acids require equilibrium calculations!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section explains how to quantify the acidity of a solution using pH and pOH scales. It introduces the ion product of water (K_w) and describes its significance in acid-base chemistry. Understanding these concepts allows for the determination of acidity and alkalinity in solutions through calculations involving concentration and dissociation constants.
Detailed
Quantitative Measures: pH, pOH, and K_w
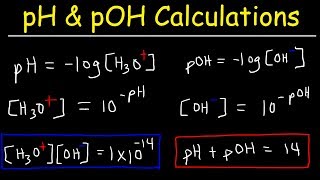
In this section, we delve into the quantitative measures used to express the acidity and basicity of solutions—namely pH, pOH, and the ion product of water, K_w. The autoionization of water is represented by the equilibrium reaction:
H₂O(l) ⇌ H⁺(aq) + OH⁻(aq)
The equilibrium constant for this reaction is called the ion product of water (K_w), which is defined as:
K_w = [H⁺][OH⁻]
At 25°C, K_w has a value of 1.0 × 10⁻¹⁴. In a neutral solution, the concentrations of hydronium ions [H⁺] and hydroxide ions [OH⁻] are equal, each being 1.0 × 10⁻⁷ M. An acidic solution is characterized by [H⁺] > [OH⁻], while a basic solution shows [OH⁻] > [H⁺].
The pH scale is a logarithmic measure of hydrogen ion concentration:
pH = -log₁₀[H⁺]
Typically ranging from 0 to 14 at 25°C, a neutral solution has a pH of 7. In contrast, the pOH scale is determined the same way for hydroxide ions:
pOH = -log₁₀[OH⁻]
A fundamental relationship exists between pH, pOH, and K_w:
pH + pOH = 14
To determine pH in strong acids and bases, the concentration can be directly used, while weak acids and bases require equilibrium calculations. Understanding these measures is crucial in acid-base chemistry, allowing for the effective analysis of solution properties.
Youtube Videos

Audio Book
Dive deep into the subject with an immersive audiobook experience.
The Ion Product of Water (K_w)
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Water itself is not entirely stable and undergoes a slight autoionization (or self-ionization), producing small amounts of hydrogen (or hydronium) ions and hydroxide ions:
H2 O(l)⇌H+(aq)+OH−(aq)
The equilibrium constant for this process is called the ion product of water, K_w:
Kw =[H+][OH−]
At a standard temperature of 25 °C, the value of K_w is 1.0 x 10$^{-14}$. It's crucial to remember that K_w is temperature-dependent.
- In a neutral solution at 25 °C, the concentrations of hydrogen and hydroxide ions are equal: [H+]=[OH−]=1.0×10−14 =1.0×10−7 M
- In an acidic solution, the concentration of hydrogen ions is greater than hydroxide ions: [H+]>[OH−].
- In a basic (alkaline) solution, the concentration of hydroxide ions is greater than hydrogen ions: [OH−]>[H+].
Detailed Explanation
K_w, or the ion product of water, describes how water molecules can split into hydrogen ions (H+) and hydroxide ions (OH−). At 25 °C, pure water will produce equal amounts of these ions, leading to both having a concentration of 1.0 x 10⁻⁷ M. However, when the solution is acidic, the concentration of H+ ions exceeds that of OH− ions, while in a basic solution, OH− ions outnumber H+ ions. This balance is crucial for understanding how acidity and basicity are determined in solutions.
Examples & Analogies
Think of K_w like the balance scale in a food truck that serves lemonade (acidic) and water (neutral). In the lemonade, there are more lemons (H+ ions), making it sour. In plain water, lemons and sugar are perfectly balanced, so it's neither sweet nor sour—just like how the concentrations of H+ and OH− are equal.
The pH Scale
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The pH scale is a logarithmic scale used to express the hydrogen ion concentration, and thus the acidity or alkalinity, of a solution in a more manageable range of numbers.
pH=−log10 [H+]
At 25 °C, the pH scale ranges typically from 0 to 14:
- Neutral solution: pH=−log10 (1.0×10−7)=7.00
- Acidic solution: pH<7.00
- Basic (alkaline) solution: pH>7.00
Detailed Explanation
The pH scale helps us understand how acidic or basic a solution is based on its H+ ion concentration. By using a logarithmic scale, we can easily express very small concentrations of hydrogen ions in a simpler way. A pH of 7 is considered neutral (like pure water), pH less than 7 indicates acidity, and pH greater than 7 indicates basicity. This makes it easier to classify different solutions.
Examples & Analogies
Consider pH like a quiz score from 0 to 14, where 7 is like passing (neutral). If you score below 7, it's like failing (more acidic), and above 7 is a strong performance (more basic or alkaline). Just like you'd adjust study habits based on your score, chemists adjust reactions based on pH levels.
The pOH Scale
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Analogous to pH, the pOH scale expresses the hydroxide ion concentration:
pOH=−log10 [OH−]
Detailed Explanation
Similar to pH, the pOH scale measures the concentration of hydroxide ions (OH−) in a solution. The relationship between pOH and pH is crucial because they combine to provide insights into the overall alkalinity or acidity of a solution.
Examples & Analogies
If pH is like tracking how many cookies you baked (amount of hydrogen ions), pOH is like keeping count of how many cookies are still in the jar (hydroxide ions). Understanding both helps ensure you have just the right amount of cookies baked!
Relationship between pH, pOH, and K_w
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
By taking the negative logarithm of the K_w expression, we derive a fundamental relationship between pH, pOH, and pK_w:
−log10 (Kw )=−log10 ([H+][OH−])−log10 (Kw )=(−log10 [H+])+(−log10 [OH−])
pKw =pH+pOH
At 25 °C, since pKw =14.00, this simplifies to:
pH+pOH=14.00
Detailed Explanation
This relationship states that the sum of pH and pOH of a solution at 25 °C is always 14. This means if we know the pH, we can easily calculate the pOH and vice versa. This relationship helps chemists understand how acidic or basic a solution is without needing to measure both values independently.
Examples & Analogies
Think of pH and pOH like two friends who always share their grades in school so that their average is 14. If one friend (pH) has a score of 9, the other friend (pOH) will need to have a 5 to keep their average at 14—just like in chemistry when you know one, you can calculate the other.
Calculations involving pH
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For Strong Acids and Bases:
- For strong acids, the concentration of H+ is directly equal to the initial concentration of the acid.
- For strong bases, the concentration of OH− is directly equal to the initial concentration of the base (for monoprotic bases like NaOH). You can then use the pH+pOH=14.00 relationship to find the pH.
For Weak Acids and Bases:
- Since weak acids and bases only partially dissociate, calculating their pH requires using their respective equilibrium dissociation constants (K_a or K_b).
- An ICE (Initial, Change, Equilibrium) table is typically employed to determine the equilibrium concentrations of H+ or OH−.
- For very weak acids/bases, or when the initial concentration is high, approximations can sometimes be made if the extent of dissociation is very small (less than 5%). Otherwise, the quadratic formula may be necessary to solve for the equilibrium concentrations.
Detailed Explanation
Calculating pH is different for strong and weak acids or bases. For strong acids, since they completely dissociate, the concentration of H+ can be directly used to find pH. In contrast, weak acids only partially break apart, so we need to apply an equilibrium approach (using an ICE table) to find their exact pH. If weak acids/bases have a very low dissociation, we can simplify calculations, but more complicated cases might require quadratic equations.
Examples & Analogies
Imagine making lemonade. If you pour a whole jug of lemon juice (strong acid), you know exactly how tangy it'll be based on how much you poured. But if you're only squeezing a few drops of lemon into water (weak acid), you have to taste (use an equilibrium table) to figure out how sour it is, because it's not all lemon—some is still just water!
Key Concepts
-
Ion Product of Water (K_w): Represents the equilibrium constant for water's autoionization.
-
pH: A logarithmic scale measuring hydrogen ion concentration, indicating acidity.
-
pOH: Related to pH; measures hydroxide ion concentration.
Examples & Applications
For a strong acid like HCl at 0.1 M, pH = 1.
For a weak acid like acetic acid at the same concentration, use ICE table to find pH from Ka.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
H and O are neat, in pure water they meet, one gives up heat, the other a seat (for H+ and OH-).
Stories
Once in a laboratory, Water realized it could split into H+ and OH-. They decided to stay equal friends, balancing their pH like bookends!
Memory Tools
Remember the phrase 'pH is the log of hydrogen - not a long process but can make you pug-nosed from its stench!'
Acronyms
Use the acronym PH4
'pH High means H+ Low
pH Low means H+ High!'
Flash Cards
Glossary
- pH
A measure of the hydrogen ion concentration in a solution, used to indicate acidity or alkalinity.
- pOH
A measure of the hydroxide ion concentration in a solution, corresponding to the pH scale.
- K_w
The ion product of water, representing the equilibrium constant for the autoionization of water.
- [H⁺]
The concentration of hydrogen ions in a solution.
- [OH⁻]
The concentration of hydroxide ions in a solution.
Reference links
Supplementary resources to enhance your learning experience.
