Defining Acids and Bases: Brønsted-Lowry and Lewis Theories
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Brønsted-Lowry Theory
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome everyone! Today, we're going to define acids and bases through the Brønsted-Lowry theory. Can anyone tell me what a Brønsted-Lowry Acid is?

Isn't it something that donates protons?

Exactly, great job, Student_1! A Brønsted-Lowry Acid donates a proton, or H⁺. Now, what about a Brønsted-Lowry Base?

It accepts protons, right?

Correct! The base accepts the proton. So, if we have a reaction, what happens to these acids and bases?

They form conjugate pairs?

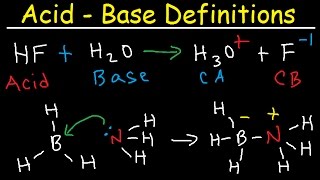
Precisely! When an acid donates a proton, it becomes a conjugate base, and when a base accepts a proton, it becomes a conjugate acid. Let's look at our first example: HCl and H₂O.

So in that case, HCl is the acid, and H₂O is the base?

Exactly! And they form Cl⁻ and H₃O⁺ as conjugates. Remember: **Acid-DONATES, Base-ACCEPTS**. This is crucial!

To recap, Brønsted-Lowry defines acids as proton donors and bases as proton acceptors, forming conjugate pairs. Any questions?
Exploring Conjugate Acid-Base Pairs
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's explore conjugate acid-base pairs a bit more. Why do you think they're important?

They help us understand the relationship between acids and bases?

That's a key insight! Conjugate pairs help us see how an acid and base are related. Let's compare HCl and Cl⁻. What happens when HCl donates its proton?

It becomes Cl⁻, which is the conjugate base?

Absolutely! And what about NH₃ in water? What does it form?

NH₄⁺ when it accepts a proton?

Brilliant! Examples like this illustrate how essential conjugate acid-base pairs are in understanding reactions. So the ruling principle is: every acid has a conjugate base, and vice versa.

Now repeat after me: **Acid donates, Base accepts. They form conjugates.** Any questions before we move on?
Introduction to Lewis Theory
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Switching gears, we now look at Lewis theory. Why do you think this theory is broader?

Because it involves electron pairs instead of just protons?

Correct! A Lewis Acid **accepts an electron pair**, while a Lewis Base **donates an electron pair**. Let's dig into examples: What makes BF₃ a Lewis acid?

Because it can accept electrons? It has an incomplete octet.

Right! Conversely, NH₃ donates its electrons. We can form an adduct here, just like in the reaction: BF₃ + NH₃.

So they form a coordinate bond?

Exactly! This is crucially significant in broader chemical contexts like complex ion formations. Remember: **Lewis Lends – Acids Take, Bases Give.**

To summarize, Lewis theory helps us include many reactions that don't involve protons. Any final thoughts?
Comparing Brønsted-Lowry and Lewis Theories
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s wrap up by comparing Brønsted-Lowry and Lewis theories. Are all Brønsted-Lowry acids also Lewis acids?

Yes, but not all Lewis acids are Brønsted-Lowry acids because they might not have protons to donate.

Excellent! This hierarchy helps solve complex chemical problems. Remember: **Every donor is a receiver, but not all receivers donate!** Can you give me examples of each?

HCl is a Brønsted-Lowry acid, and BF₃ is a Lewis acid that can't donate protons!

Perfect! You've grasped the nuances well. In summary, Brønsted-Lowry focuses on protons while Lewis expands to electron pairs. Keep this distinction clear!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Acids and bases can be defined through various theories, most notably the Brønsted-Lowry and Lewis theories. Brønsted-Lowry focuses on proton donation and acceptance, forming conjugate acid-base pairs. In contrast, Lewis theory broadens the definitions to include electron pair donation and acceptance, illustrating a wider range of acid-base interactions beyond proton transfer.
Detailed
Defining Acids and Bases: Brønsted-Lowry and Lewis Theories
In the study of acids and bases, understanding their definitions and behaviors is fundamental to grasping chemical interactions. This section introduces two prominent theories:
Brønsted-Lowry Theory
The Brønsted-Lowry theory, widely utilized in general chemistry and foundational in the IB Chemistry curriculum, defines:
- Brønsted-Lowry Acid: A species that donates a proton (H⁺).
- Brønsted-Lowry Base: A species that accepts a proton (H⁺).
In any Brønsted-Lowry acid-base reaction, protons are transferred from the acid to the base. This transfer is crucial as it leads to the formation of conjugate pairs:
- When an acid donates a proton, the resulting species is called its conjugate base.
- Conversely, when a base accepts a proton, the resulting species is termed its conjugate acid.
Examples of Conjugate Acid-Base Pairs:
- HCl (acid) + H₂O (base) ⇌ Cl⁻ (conjugate base) + H₃O⁺ (conjugate acid)
- NH₃ (base) + H₂O (acid) ⇌ NH₄⁺ (conjugate acid) + OH⁻ (conjugate base)
Water can act as both an acid and a base, which makes it amphiprotic. Other notable amphiprotic species include bicarbonate (HCO₃⁻) and dihydrogen phosphate (H₂PO₄⁻).
Lewis Theory
The Lewis theory offers a more inclusive framework for defining acids and bases without needing proton transfer:
- Lewis Acid: A species that accepts an electron pair (often electron-deficient species, e.g., cations like Fe³⁺ or compounds with incomplete octets such as BF₃).
- Lewis Base: A species that donates an electron pair (electron-rich species with lone pairs like NH₃ and OH⁻).
In a Lewis acid-base reaction, a dative bond forms between the Lewis base and the Lewis acid, resulting in an adduct.
Examples of Lewis Acid-Base Reactions:
- BF₃ (Lewis acid) + NH₃ (Lewis base) → F₃B-NH₃ (adduct)
- H⁺ (Lewis acid) + OH⁻ (Lewis base) → H₂O
- Fe³⁺ (Lewis acid) + 6CN⁻ (Lewis base) → [Fe(CN)₆]³⁻ (complex ion)
It is important to note that all Brønsted-Lowry acids and bases are also considered Lewis acids and bases, but not vice versa. For example, BF₃ acts as a Lewis acid since it accepts an electron pair, but it does not qualify as a Brønsted-Lowry acid as it cannot donate protons.
Youtube Videos

Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Acid-Base Definitions
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The understanding of acids and bases has evolved to encompass a broader spectrum of chemical behavior.
Detailed Explanation
Acids and bases are essential concepts in chemistry. Initially, we had narrow definitions for them, but the scientific community broadened our understanding. The Brønsted-Lowry and Lewis theories represent two key approaches to defining acids and bases. These theories help explain the behavior of these substances in a variety of chemical reactions.
Examples & Analogies
Think of acids and bases like two friends in a game of catch—each has something valuable to offer. The Brønsted-Lowry theory describes how they pass a ball (protons), while the Lewis theory describes how they share their resources (electron pairs). Understanding these interactions is crucial, just like knowing how your friends play can improve teamwork.
The Brønsted-Lowry Theory
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This is the most widely adopted definition in general chemistry and forms the basis for much of the IB Chemistry curriculum.
● Brønsted-Lowry Acid: A species that donates a proton (H$^+$).
● Brønsted-Lowry Base: A species that accepts a proton (H$^+$).
In any Brønsted-Lowry acid-base reaction, protons are transferred from the acid to the base. This transfer leads to the formation of conjugate acid-base pairs:
● When an acid donates a proton, the remaining species is its conjugate base.
● When a base accepts a proton, the newly formed species is its conjugate acid.
Detailed Explanation
The Brønsted-Lowry theory defines acids as proton donors and bases as proton acceptors. When an acid donates a proton, it creates a conjugate base, whereas when a base accepts a proton, it becomes a conjugate acid. For example, in a reaction involving hydrochloric acid and water, HCl donates a proton to water, forming the conjugate base Cl⁻ and the conjugate acid H₃O⁺. This conceptual framework helps to track the flow of protons in chemical reactions.
Examples & Analogies
Imagine a game where players can only pass balls to each other. If Player A (the acid) throws a ball to Player B (the base), Player B now has one more ball than before (the conjugate acid). The ball Player A had last is now with Player B (the conjugate base). This way of thinking about acids and bases helps visualize how they interact in various chemical reactions.
Conjugate Acid-Base Pairs
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
These two species always differ by exactly one proton.
Examples of Conjugate Acid-Base Pairs:
● Example 1: Hydrochloric Acid (Acid) in Water (Base) HCl (acid) + H₂O (base) ⇌ Cl⁻ (conjugate base) + H₃O⁺ (conjugate acid)
● Example 2: Ammonia (Base) in Water (Acid) NH₃ (base) + H₂O (acid) ⇌ NH₄⁺ (conjugate acid) + OH⁻ (conjugate base)
Detailed Explanation
Conjugate acid-base pairs are foundational for understanding acid-base chemistry. Each pair consists of an acid and its corresponding base, which differ by one proton. For example, hydrochloric acid (HCl) donates a proton to water (H₂O) to form the conjugate base (Cl⁻) and the conjugate acid (H₃O⁺). Likewise, ammonia (NH₃) can accept a proton from water and become the conjugate acid (NH₄⁺), while water now becomes its conjugate base (OH⁻). This relationship shows how acids and bases interact with their surroundings.
Examples & Analogies
Think of a relay race where runners pass a baton. The runner who starts with the baton represents the acid and the runner switching out for the baton represents the base. Once the baton is passed, the new runner becomes the 'conjugate' runner who previously didn't have the baton, reflecting how potential energy (in this case, protons) is transferred during chemical reactions.
The Lewis Theory
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The Lewis theory offers a broader and more general definition, particularly useful for reactions that do not involve the transfer of protons.
● Lewis Acid: A species that accepts an electron pair.
● Lewis Base: A species that donates an electron pair. In a Lewis acid-base reaction, a coordinate (dative) covalent bond is formed between the electron pair donor (Lewis base) and the electron pair acceptor (Lewis acid). The resulting product is often called an adduct.
Detailed Explanation
The Lewis theory defines acids and bases based on their electron behavior. A Lewis acid is an electron pair acceptor, while a Lewis base is an electron pair donor. In a Lewis acid-base reaction, these species form a coordinate bond— where the Lewis base donates an electron pair to the Lewis acid. For example, when ammonia (NH₃), acting as a Lewis base, donates an electron pair to boron trifluoride (BF₃), a Lewis acid, they form an adduct (F₃B-NH₃). This theory is particularly useful in understanding complex reactions that involve no protons.
Examples & Analogies
Consider a situation where two friends are collaborating on a project. One friend (the Lewis base) has a lot of ideas (electron pairs) to offer, while the other friend (the Lewis acid) is looking for assistance to enhance his project (accepting electron pairs). Together, they create something new—just as Lewis acids and bases combine to form new compounds.
Differences Between Brønsted-Lowry and Lewis Theories
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
It's important to note that all Brønsted-Lowry acids and bases are also Lewis acids and bases, but the converse is not always true. For example, boron trifluoride (BF₃) is a Lewis acid because it accepts an electron pair, but it's not a Brønsted-Lowry acid as it has no proton to donate.
Detailed Explanation
While all Brønsted-Lowry acids and bases fit into the Lewis definition, not all Lewis acids and bases fit into the Brønsted-Lowry system. For example, boron trifluoride (BF₃) can accept electron pairs, making it a Lewis acid, but it does not donate protons, meaning it does not qualify as a Brønsted-Lowry acid. This distinction highlights the broader applicability of the Lewis theory in chemical reactions.
Examples & Analogies
Imagine a class where everyone is either a helper (electron donor) or someone who needs help (electron acceptor). All students who help can also fit in the category of those who can provide tangible support. However, some students may only need help but can’t aid anyone else. In chemistry, this illustrates how the broader Lewis definition encompasses more scenarios than the specific Brønsted-Lowry definition.
Key Concepts
-
Brønsted-Lowry Theory: Focuses on proton donation and acceptance.
-
Conjugate Acid-Base Pairs: Important for understanding the relationship between acids and bases.
-
Lewis Theory: Broadens definitions to include electron pair interactions.
-
Amphiprotic Species: Substances that can act both as acids and bases.
Examples & Applications
HCl (acid) + H₂O (base) ⇌ Cl⁻ (conjugate base) + H₃O⁺ (conjugate acid)
NH₃ (base) + H₂O (acid) ⇌ NH₄⁺ (conjugate acid) + OH⁻ (conjugate base)
Water can act as both an acid and a base, which makes it amphiprotic. Other notable amphiprotic species include bicarbonate (HCO₃⁻) and dihydrogen phosphate (H₂PO₄⁻).
Lewis Theory
The Lewis theory offers a more inclusive framework for defining acids and bases without needing proton transfer:
Lewis Acid: A species that accepts an electron pair (often electron-deficient species, e.g., cations like Fe³⁺ or compounds with incomplete octets such as BF₃).
Lewis Base: A species that donates an electron pair (electron-rich species with lone pairs like NH₃ and OH⁻).
In a Lewis acid-base reaction, a dative bond forms between the Lewis base and the Lewis acid, resulting in an adduct.
Examples of Lewis Acid-Base Reactions:
BF₃ (Lewis acid) + NH₃ (Lewis base) → F₃B-NH₃ (adduct)
H⁺ (Lewis acid) + OH⁻ (Lewis base) → H₂O
Fe³⁺ (Lewis acid) + 6CN⁻ (Lewis base) → [Fe(CN)₆]³⁻ (complex ion)
It is important to note that all Brønsted-Lowry acids and bases are also considered Lewis acids and bases, but not vice versa. For example, BF₃ acts as a Lewis acid since it accepts an electron pair, but it does not qualify as a Brønsted-Lowry acid as it cannot donate protons.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
An acid gives a proton, that’s the way it flows, / A base will take it back, just like everyone knows.
Stories
Imagine a battle where acids throw protons like balls, and bases catch them, forming conjugate pairs, heroes that rise, ensuring the chemistry never stalls!
Memory Tools
To remember Brønsted-Lowry: AB – Acid Bonkers; Base is its Assistant.
Acronyms
Remember
**LEAD** - Lewis Acids Accept Donors.
Flash Cards
Glossary
- BrønstedLowry Acid
A species that donates a proton (H⁺).
- BrønstedLowry Base
A species that accepts a proton (H⁺).
- Conjugate Acid
The species formed when a base accepts a proton.
- Conjugate Base
The species formed when an acid donates a proton.
- Lewis Acid
A species that accepts an electron pair.
- Lewis Base
A species that donates an electron pair.
- Adduct
The resultant compound formed from the reaction of a Lewis acid and a Lewis base.
- Amphiprotic
A substance that can act as both an acid and a base.
Reference links
Supplementary resources to enhance your learning experience.
