Energy
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Energy and Frequency of Light
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’re going to delve into the energy of light. Who can tell me what we mean by the energy of light?

Is it related to how bright the light is?

Great question! Energy is actually related to both brightness and frequency. The higher the frequency, the more energy the light has. This relationship is given by an important equation—you might remember it as E equals h times f.

What does 'h' stand for in that equation?

Excellent! 'h' represents Planck’s constant, which is a very small number but crucial in quantum mechanics. Can anyone tell me why this concept is important in optoelectronics?

Because it helps us understand how light interacts with materials!

Exactly! The energy from light affects how electrons in materials behave, which is vital for devices like solar cells and LEDs. Remember: **E = h ⋅ f** can help you recall the link between energy and frequency.

So does it mean higher frequency light is more energetic? Like UV light compared to red light?

That's correct! UV light has a higher frequency and therefore more energy than red light. Excellent participation, everyone! To recap, light's energy is determined by its frequency, described by the equation E = h ⋅ f.
Photon Concept
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's talk about photons. Who can explain what a photon is?

Isn’t it a small particle of light that carries energy?

Exactly! A photon is indeed a particle of light. It carries energy based on its frequency. If we combine our earlier discussions, what happens if the frequency increases?

The energy increases as well!

Right! So as we think about designing devices for optoelectronics, what implications can we draw from this relationship between photons and energy?

Higher energy light can excite more electrons, which is essential for devices like LEDs!

Exactly, and in photovoltaic cells, higher energy photons can effectively generate electricity. Can anyone summarize how energy relates back to light interactions?

Energy determines what happens when light hits a material, such as absorption or emission!

Perfect summary! So always remember that energy is at the heart of how light interacts with materials.
Light-Matter Interaction Examples
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To connect our concepts to real-world applications, can anyone give an example of where light energy is applied in technology?

Solar panels convert light into energy!

Yes! That’s an excellent example. When light hits the solar cell, its energy excites electrons, generating electrical current. What about other devices?

LED lights! They use energy to create light by exciting electrons.

Absolutely! LEDs rely on the energy from photons to emit light when electrons drop to lower energy levels. Any other applications you can think of?

There are lasers too! They use light energy to produce concentrated beams.

Correct! Lasers utilize stimulated emission, which is an exciting application in medicine and telecommunications. Let’s summarize what we learned today regarding light energy and its interactions: energy determines how light behaves when it meets different materials, leading to diverse applications.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, the energy of light is discussed in terms of its frequency and the equation that relates the two, highlighting the role of photons. Understanding this relationship is essential for grasping how light interacts with materials, forming the foundation for optoelectronic devices.
Detailed
Energy of Light in Optoelectronics
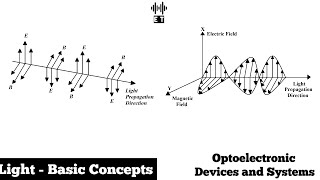
In the study of light and its interaction with materials, energy holds significant importance, especially in the field of optoelectronics. Light can be modeled both as a wave and as a particle (photon), and its energy is directly proportional to its frequency. The energy (E) can be calculated using the equation:
E = h ⋅ f,
where h is Planck’s constant and f is the frequency of the light wave. This relationship shows that as the frequency of light increases, so does its energy, which directly impacts how it interacts with various materials.
For optoelectronic devices, this energy determines processes like absorption, reflection, emission, and scattering. For example, when light is absorbed by a material, it can excite electrons to higher energy states, crucial for devices like solar cells. Understanding these interactions not only provides insight into the functionality of various optoelectronic devices but also informs the design and engineering of new technologies. The dual wave-particle nature of light, encapsulated through the concept of energy, allows for a versatile application across numerous fields including telecommunications, healthcare, and renewable energy.
Youtube Videos


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Energy in Light
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Light exhibits both particle-like and wave-like behavior. As a particle (photon), light has energy given by E=h⋅f, where h is Planck’s constant. The energy of light determines how it interacts with matter.
Detailed Explanation
This chunk introduces the concept of energy in relation to light. Light behaves as both a wave and a particle. As a particle, it is called a photon, and its energy can be calculated using the equation E = h·f, where E represents energy, h (Planck’s constant) is a fundamental constant in physics, and f is the frequency of light. The energy of light is significant because it influences how light interacts with different materials; for instance, higher-energy light (like ultraviolet) can cause electrons in a material to move, while lower-energy light (like infrared) may not have enough energy to cause such effects.
Examples & Analogies
Think of light energy like a basketball being thrown. The faster you throw the basketball (higher frequency), the more energy it has, and it can tower over other objects more easily. Similarly, light with a higher frequency can interact with materials in more energetic ways.
Photons and Their Energy
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A photon is a quantum of light, which behaves like a particle. Photons carry energy proportional to their frequency, but they do not have mass.
Detailed Explanation
This chunk focuses on photons, which are the fundamental particles of light. Photons are unique because they possess energy that is directly related to their frequency, meaning that as frequency increases, so does the energy of the photon. Despite their energy, photons are massless, which allows them to travel at the speed of light in a vacuum. Understanding that photons are both energy carriers and massless is crucial for grasping concepts in optoelectronics, such as how they can induce electrical changes in a material without weight or resistance slowing them down.
Examples & Analogies
Imagine photons as tiny invisible cars zooming around in a race. Each car travels at the same speed (the speed of light), but some cars have different colors representing different frequencies—red is slow and has less energy, while blue is faster and more energetic. Just like in a race, the faster the car, the more impact it can make when it reaches its goal.
Key Concepts
-
Energy is directly related to the frequency of light;
-
Photons are particles of light that carry energy;
-
Planck's constant explains the relationship between energy and frequency.
Examples & Applications
The operation of solar cells relies on the absorption of photons, which generate electrical current.
LEDs emit light through the process of electron recombination when excited by photon energy.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Light's energy shines, as frequency climbs, Planck’s constant helps us determine the primes.
Stories
Once upon a time, in Quantum Land, a little photon wanted to play. It danced on frequencies, high and low, its energy showed the way.
Memory Tools
Remember 'E equals hf' to connect energy to frequency effortlessly!
Acronyms
PEP stands for Photon Energy Proportional—indicating that photons have energy related to their frequency.
Flash Cards
Glossary
- Energy
The capacity to perform work, measured in joules, associated with the frequency of light.
- Photon
A particle of light that carries energy proportional to its frequency.
- Planck’s constant
A fundamental constant (approximately 6.626 x 10^-34 J·s) that relates energy and frequency in quantum mechanics.
Reference links
Supplementary resources to enhance your learning experience.
