Frequency (f)
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Frequency
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will dive into the concept of frequency. Can anyone tell me what frequency is?

Isn't it related to how fast something vibrates?

Exactly! Frequency refers to the number of cycles that occur in a second, which in terms of light, helps us understand its behavior and energy. To remember, think of F for Frequency and F for Fast!

So, if a light wave has a higher frequency, does that mean it has more energy?

Yes! Higher frequency equates to higher energy. This is expressed in the formula E = h * f. Can anyone explain how this might apply to an LED?

An LED emits light when electrons fall to a lower energy level, producing photons with specific frequencies, right?

Exactly! So, frequency is crucial in determining the kind of light an LED emits.

Let's summarize: Frequency tells us how many cycles of light pass per second and influences energy!
Frequency and Wavelength Relationship
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's connect frequency with wavelength. Who can tell me how these concepts are related?

I think they are inversely related, right?

Correct! As frequency increases, wavelength decreases. To help you remember this, consider the acronym 'F.In.W.' — Frequency Inverse with Wavelength.

So, if I see a wave with a high frequency, that means it has a short wavelength?

Absolutely! This relationship is key in understanding how light interacts with materials. For example, in fiber optics, different frequencies and wavelengths will behave differently.

How does this relate to prisms and colors?

Great question! When light passes through a prism, different wavelengths refract by different amounts—creating the spectrum of colors we see. Remember, light's wavelength and frequency determine its characteristics!

So we learned that frequency and wavelength are inversely related and crucial for applications like fiber optic technology.
Practical Applications of Frequency in Optoelectronics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's discuss how frequency applies to different optoelectronic devices, starting with solar cells!

How does frequency affect how solar cells generate electricity?

Excellent! Solar cells absorb light, and the frequency of that light influences how effectively they can excite electrons. Higher-frequency light generates more energetic electrons, enhancing efficiency.

And what about laser diodes?

For laser diodes, the frequency determines the color of the light emitted. Different frequencies emit different colors, enabling lasers to be used in varied applications, from medical to telecommunications.

Can we relate this to energy consumption?

Definitely! Understanding the frequency of light ensures that devices are optimized for energy efficiency. Remember: F for frequency, E for energy — they are integral to our devices.

Let’s recap: Frequency impacts solar cells’ efficiency and the color from laser diodes, showing the importance of this concept in practical applications!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
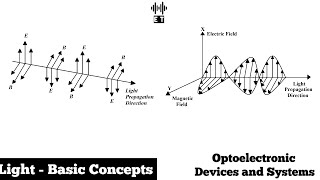
In the study of light, frequency (f) indicates how many cycles of a wave pass a point in one second. It's directly related to the energy of light, where higher frequency corresponds to higher energy. Understanding frequency is crucial for applications in optoelectronic devices.
Detailed
In the context of optoelectronics, frequency (f) is a pivotal concept, reflecting the number of cycles of light that pass a given point in one second. This property is inversely related to wavelength (λ); as the frequency increases, the wavelength conversely decreases. The energy associated with light corresponds to its frequency, described by the equation E = h * f, where h is Planck’s constant. Therefore, light waves of higher frequency possess more energy, influencing their interactions with materials. In optoelectronic devices, such as LEDs and solar cells, fully understanding frequency's implications aids in optimizing performance and efficiency in light-based technologies.
Youtube Videos


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Frequency
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Frequency (f): The number of cycles of the wave that pass a point in one second.
Detailed Explanation
Frequency is a measure of how often an event occurs in a specific time period. In the context of waves, it refers to the number of complete cycles of the wave that pass a point in one second. For example, if a wave makes 5 full cycles in one second, we say its frequency is 5 hertz (Hz). This concept helps us understand how quickly waves oscillate.
Examples & Analogies
Think of a swing at a playground. Every time the swing goes back and forth is one complete cycle. If you time how many times the swing goes back and forth in a minute, you’re measuring its frequency, and it might be similar to counting how many waves hit the shore in the same time frame.
Frequency and Wavelength Relationship
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Frequency is related to energy by the equation E=h⋅f, where h is Planck’s constant.
Detailed Explanation
This relationship shows that frequency is directly linked to the energy of the light. Planck’s constant (h) is a fundamental constant in physics that provides a proportionality factor. If we increase the frequency of light (make it oscillate faster), its energy also increases according to the equation. Thus, the higher the frequency, the more energetic the light.
Examples & Analogies
Imagine a water fountain. If you increase the speed at which the water shoots up (analogous to increasing frequency), the water will shoot higher, indicating more energy. Similarly, in light, when the frequency increases, the energy of the light also increases, which is why higher frequency light is more effective at exciting electrons.
Photon and Frequency Link
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
As a particle (photon), light has energy given by E=h⋅f. The energy of light determines how it interacts with matter.
Detailed Explanation
In this context, a photon is considered a particle of light, and its energy is defined by the same equation involving frequency. Thus, photons with higher frequency can cause different interactions with materials, such as ionization, where they can knock electrons off atoms. The specific interactions depend on the energy of the incoming photons, which is determined by their frequency.
Examples & Analogies
Picture a soccer ball (photon) being kicked at different speeds (frequencies). If you kick it softly, it moves slowly (low frequency), but if you kick it hard, it flies quickly and can knock down a player (high frequency), showing how energy can change the reaction based on how fast the ball travels.
Key Concepts
-
Frequency (f): The number of cycles of light wave per second, related to energy.
-
Wavelength (λ): The distance between two successive peaks of a wave, inversely related to frequency.
-
Photon: The energy packet of light, carrying energy related to its frequency.
Examples & Applications
In fiber optics, the transmission of data relies on understanding how different frequencies of light behave within the fiber.
In solar cells, the efficiency of energy conversion is impacted by the frequency of light absorbed, with higher frequencies generating more electric current.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Frequency's fast, energy is vast; Waves high and low, in rhythms they flow.
Stories
Imagine a race between waves—those that run fast (high frequency) are brighter and more energetic, illuminating the dark.
Memory Tools
F.E.W: Frequency, Energy, Wavelength—remember, as frequency goes up, energy goes up, and wavelength goes down.
Acronyms
F.I.W
Frequency Inversely With Wavelength
reminder of their relationship.
Flash Cards
Glossary
- Frequency (f)
The number of cycles of a wave that pass a point in one second, related to energy through E = h * f.
- Photon
A quantum of light, exhibiting both wave and particle properties, associated with a specific frequency and energy.
- Wavelength (λ)
The distance between consecutive peaks or troughs of a light wave, inversely related to frequency.
Reference links
Supplementary resources to enhance your learning experience.
