The Physics of Light
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Properties of Light: Wavelength and Frequency
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are going to explore the properties of light, starting with wavelength and frequency. Can anyone tell me what wavelength is?

Isn't wavelength the distance between two peaks of a wave?

Exactly! And can someone tell me how wavelength relates to frequency?

They are inversely related, right? As one increases, the other decreases?

Correct! If you remember the acronym 'WAVE' for Wavelength and Frequency, it’ll help you recall how they interact. Let’s connect that to how wavelength determines color.

So, shorter wavelengths would mean blue light and longer wavelengths mean red light?

Exactly! Let’s recap: Wavelength is the distance between peaks, and frequency is the number of cycles per second. They are inversely related, affecting the color of light.
Photon Properties and Energy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss photons. What is a photon?

Isn't it a particle of light that has energy but no mass?

That's right! The energy of a photon is given by the formula E=h⋅f. Can someone explain what 'h' represents?

It’s Planck’s constant!

Exactly! Remember, 'H for High Energy'. The higher the frequency, the higher the energy. Can anyone give an example of how this interaction with materials plays out?

In solar cells, higher energy photons can excite electrons for electricity!

Great observation! So to summarize, photons are massless particles of light, and their energy is proportional to their frequency, influencing materials’ interactions.
Interactions of Light with Materials
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's talk about how light interacts with materials. First, what happens during absorption?

When light hits a material, it can be absorbed, which excites the electrons to a higher state.

Good! And how does this apply in devices like solar cells?

Solar cells convert sunlight into electricity by absorbing photons!

Exactly! Now, who can explain what reflection and refraction are?

Reflection is when light bounces off a surface, and refraction is when light bends as it moves into a different medium!

Perfect! And these principles are crucial for lenses and optical fibers. Now, can someone share what scattering is?

Scattering is when light is diverted as it passes through materials, affecting how we perceive colors!

Well done! Let's recap the four interactions: absorption, reflection, refraction, and scattering, each vital for optoelectronic technologies.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, we delve into the nature of light as electromagnetic radiation, discussing key properties such as wavelength, frequency, energy, and photons. We also examine how light interacts with materials through phenomena like absorption, reflection, refraction, emission, and scattering, all of which are crucial for the functioning of optoelectronic devices.
Detailed
The Physics of Light
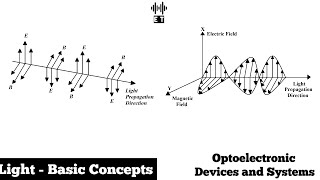
Light is a form of electromagnetic radiation that travels through space at a speed of approximately 3 × 10^8 meters per second in a vacuum, exhibiting both wave-like and particle-like properties. This section identifies and explains the key properties of light:
- Wavelength (λ): The distance between consecutive peaks of a light wave, inversely related to frequency, determining the color in the visible spectrum.
- Frequency (f): The number of cycles that pass a given point per second, connected to light's energy via the equation E=h⋅f, where h is Planck’s constant.
- Energy: Light behaves as photons. The energy of a photon is determined by its frequency, influencing how it interacts with matter.
- Photon: A photon is a quantum of light without mass, carrying energy based on its frequency.
Interactions of light with materials include absorption, reflection, refraction, emission, and scattering, which are pivotal in optoelectronic applications:
- Absorption: Light entering a material may excite electrons, vital for the functioning of solar cells and photodiodes.
- Reflection and Refraction: Light can be reflected or bent when transitioning between media, important for optical fibers and lenses.
- Emission: Electrons returning to lower energy states can emit photons, key for LEDs and laser diodes.
- Scattering: The deviation of light as it passes through a material, influencing intensity and quality, crucial for fiber optics and other light-transmitting devices.
Understanding these principles is essential for innovations in optoelectronic technologies.
Youtube Videos


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Light
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Light is an electromagnetic wave that propagates through space at a speed of approximately 3 × 10^8 meters per second in a vacuum.
Detailed Explanation
Light can be defined as an electromagnetic wave, meaning it is a form of energy that travels through space. One key characteristic of light is its speed; in a vacuum, it moves at about 300 million meters per second. This is one of the fastest speeds in the universe and highlights how quickly light can travel across large distances, such as from the Sun to the Earth, taking only about 8 minutes.
Examples & Analogies
Think of how fast light travels like a lightning bolt during a thunderstorm. When you see lightning, you might notice that you hear the thunder some seconds later. This is similar to how light from stars travels vast distances to reach us, often taking years to reach Earth.
Key Properties of Light
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The key properties of light include:
• Wavelength (λ): The distance between two consecutive peaks (or troughs) of a light wave. Wavelength is inversely related to frequency and determines the color of light in the visible spectrum.
• Frequency (f): The number of cycles of the wave that pass a point in one second. Frequency is related to energy by the equation E=h⋅f, where h is Planck’s constant.
• Energy: Light exhibits both particle-like and wave-like behavior. As a particle (photon), light has energy given by E=h⋅f. The energy of light determines how it interacts with matter.
• Photon: A photon is a quantum of light, which behaves like a particle. Photons carry energy proportional to their frequency, but they do not have mass.
Detailed Explanation
Light possesses several essential properties that help us understand its behavior and characteristics:
1. Wavelength refers to the size of the wave; it's the distance between peaks or troughs. It affects the color we see; shorter wavelengths correspond to blue and violet colors while longer wavelengths correlate with red.
2. Frequency indicates how often the wave oscillates per second. Higher frequency waves have more energy. According to the equation E = h·f (where h is Planck's constant), light with a higher frequency carries more energy.
3. Energy relates to how light interacts with other materials in terms of absorption and emission; photons can excite electrons, leading to different outcomes in various materials.
4. Photons are the fundamental units of light. Unlike other particles, they have no mass. Their energy is directly related to their frequency, and every time a photon interacts with matter, it can cause different physical effects based on their energy levels.
Examples & Analogies
Imagine light as a group of surfers riding ocean waves. The height of each wave correlates with the surfer’s frequency of riding up and down. Taller waves (high wavelength) represent red light whereas smaller ones (low wavelength) illustrate blue light. Photons are like individual surfers, with some able to catch the bigger waves and perform stunts (higher frequencies) while others might just glide along the surface (lower frequencies).
Interactions of Light with Materials
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When light encounters a material, several interactions can occur, depending on the properties of the material and the wavelength of the light. These interactions are crucial for the operation of optoelectronic devices.
• Absorption: When light enters a material, it may be absorbed, exciting electrons to higher energy states. This is the fundamental process that enables photovoltaic devices (like solar cells) and photodiodes to function.
• Reflection and Refraction: Light can be reflected off a surface or refracted (bent) when passing from one medium to another. These interactions are important in devices like optical fibers and lenses.
• Emission: When electrons in a material return to a lower energy state, they can emit photons. This phenomenon underlies the operation of LEDs and laser diodes.
• Scattering: Light can be scattered as it passes through a material, affecting the intensity and quality of the light. Scattering is important in the design of devices that rely on light transmission, such as fiber optics.
Detailed Explanation
Light can interact with materials in four significant ways to create different phenomena:
1. Absorption occurs when light energy is taken in by a material, which causes its electrons to move to higher energy levels. This is what enables devices like solar panels to convert light into usable electrical energy.
2. Reflection and Refraction happen when light encounters a boundary between two different media, like air and glass. Reflection is when light bounces off a surface, while refraction is the bending of light as it passes into a denser medium—these principles are what allow lenses to focus light and are essential for fiber optics.
3. Emission is the process by which materials release energy in the form of photons when excited electrons return to their ground state. This is how LEDs emit visible light.
4. Scattering is when particles in a material cause light to deviate from its original path, which can change the color and brightness of the transmitted light. It's a principle seen in the blue sky, where shorter wavelengths scatter more than longer ones, giving the sky its blue hue.
Examples & Analogies
Consider a water surface on a sunny day. When sunlight hits the water, some light reflects off the surface and some penetrates into the water, bending as it goes deeper (refraction). In this case, the water absorbs certain colors, changing the overall color you see. A very similar process occurs in solar panels, where sunlight is absorbed to create energy, reflecting the idea of turning light into usable power.
Key Concepts
-
Wavelength: The distance between peaks of a light wave, determining color.
-
Frequency: The number of wave cycles per second, related to energy.
-
Photon: A massless particle of light with energy proportional to its frequency.
-
Absorption: Light being taken up by materials, vital for solar cells.
-
Reflection: The bouncing of light from surfaces.
-
Refraction: The bending of light as it passes into different mediums.
-
Emission: Release of photons when electrons fall to lower energy states.
-
Scattering: The scattering of light affecting intensity during transmission.
Examples & Applications
In fiber optics, light is refracted to transmit information over long distances.
LEDs operate through photon emission, converting electrical energy into light.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
To remember light, think of the bright day, Wavelength and frequency lead the way.
Stories
Imagine a photon dancing to its frequency, bringing color to our world while being light as air.
Memory Tools
Use the acronym 'PARE' to recall Photons, Absorption, Reflection, Emission.
Acronyms
WAVE represents Wavelength, Absorption, Velocity, Energy.
Flash Cards
Glossary
- Wavelength (λ)
The distance between two consecutive peaks of a light wave, inversely related to frequency.
- Frequency (f)
The number of cycles of a light wave that pass a point in one second, related to energy by E=h⋅f.
- Energy
The capacity of light to do work, determined by its frequency, given by the formula E=h⋅f.
- Photon
A massless particle of light that carries energy proportional to its frequency.
- Absorption
The process by which light is taken up by a material, exciting its electrons.
- Reflection
The bouncing back of light when it hits a surface.
- Refraction
The bending of light as it passes from one medium to another.
- Emission
The release of photons when electrons return to a lower energy state.
- Scattering
The deflection of light as it passes through a material, affecting its intensity and quality.
Reference links
Supplementary resources to enhance your learning experience.
