Photon
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Photons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to learn about photons. A photon is essentially a quantum of light, meaning it's the smallest possible unit of electromagnetic radiation. Who can tell me what they think the properties of a photon are?

I think photons are massless particles, right?

Exactly! Photons don't have any mass. Along with that, they carry energy that's proportional to their frequency. We can express this relationship with an equation: E = h·f. Can anyone explain what each variable means?

E is energy, h is Planck’s constant, and f is frequency?

Spot on! Remember, higher frequency means higher energy. Let's remember that using the mnemonic 'High Frequency, High Energy.'

Does that mean different colors of light have different energy levels because they have different frequencies?

Yes, precisely! That's why light appears to have different colors based on its wavelength.

To sum up, a photon is a massless particle that carries energy, proportional to its frequency, which significantly influences its interactions with materials.
Interactions of Photons with Materials
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we've defined a photon, let's talk about how photons interact with materials. Can anyone name the interactions we discussed?

I remember absorption and emission are two of them!

Great recall! Absorption is when a photon excites electrons in a material to higher energy states. Can anyone give me an example of where this happens?

In solar cells, right? Photons get absorbed and generate electricity.

Correct! Other interactions include reflection and refraction. Can someone explain those concepts?

Reflection is when light bounces off a surface, and refraction is when it bends while passing into another medium.

That’s right! To help us remember these concepts, let's use the acronym 'ARRR' – **A**bsorption, **R**eflection, **R**efraction, **E**mission. This way, we can easily recall the primary interactions.

What about scattering?

Scattering is also important! It affects the intensity and quality of light. For example, this is crucial in the design of fiber optics. In summary, photons interact with materials primarily through absorption, reflection, refraction, emission, and scattering.
Importance of Photons in Optoelectronics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's explore why understanding photons is essential for optoelectronics. What do you think? Why do we care about photons in this field?

Photons are how we generate and detect light!

Exactly! Devices like LEDs and solar cells rely on photon interactions. Can anyone summarize how a solar cell works?

When light hits the solar cells, photons are absorbed, exciting electrons to create an electrical current.

Great explanation! Remember, it's the absorption and emission of photons that drive the operation of these devices. The concept of energy transfer is key.

And LEDs emit light when electrons fall back to lower energy states releasing photons, right?

Absolutely! So, to summarize this importance: Photons are crucial for the operation of optoelectronic devices, influencing how we generate, detect, and use lighting in our technology.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section covers the definition of a photon as a quantum of light, including its properties such as energy, frequency, and behavior. It emphasizes the role of photons in the interaction of light with materials and their importance in modern optoelectronic devices.
Detailed
Detailed Summary
The term photon refers to a quantum of light, exhibiting behavior characteristic of both waves and particles, a phenomenon described by wave-particle duality. Photons are massless and carry energy that is directly proportional to their frequency, illustrated by the equation E = h·f, where E represents energy, h is Planck’s constant, and f denotes frequency. This relationship is fundamental in understanding how light interacts with materials, particularly semiconductors, which are crucial for optoelectronic devices like LEDs, solar cells, and photodiodes.
Furthermore, photons are responsible for various interactions with materials, such as absorption, reflection, refraction, emission, and scattering. Understanding these interactions is vital for developing and improving optoelectronic technology.
Youtube Videos


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of a Photon
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A photon is a quantum of light, which behaves like a particle. Photons carry energy proportional to their frequency, but they do not have mass.
Detailed Explanation
A photon is the fundamental unit or 'quantum' of light. Unlike many particles that we encounter in daily life, photons are interesting because they don't have any mass. Instead, they represent a 'packet' of energy that can be described as both a wave and a particle. Their energy is directly linked to their frequency; as the frequency increases, so does the energy of the photon.
Examples & Analogies
Think of a photon like a small, invisible ball of energy traveling through space. Just as a tennis ball's speed and force depend on how hard it is thrown, a photon’s 'energy' depends on its 'frequency.' Higher-frequency light, like blue light, has more energy than lower-frequency light, like red light, just as a faster tennis ball can hit harder.
Properties of Photons
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Photons carry energy proportional to their frequency, but they do not have mass.
Detailed Explanation
Photons have a unique role in the physics of light; they are massless carriers of electromagnetic energy. Instead of having mass, they possess energy that can be calculated using the relationship E = h⋅f, where 'E' is the energy, 'h' is Planck's constant, and 'f' represents frequency. This relationship signifies that as photons change in frequency, their energy changes in direct relation.
Examples & Analogies
Imagine you are in a swimming pool, where the waves represent different frequencies of light. When smaller waves (lower frequencies) pass by, they gently ripple the water. But when larger waves (higher frequencies) come through, they crash more forcefully against the sides of the pool, representing the increased energy of higher-frequency photons.
Photon Behavior
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
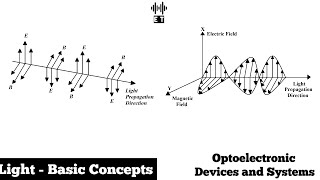
Light exhibits both particle-like and wave-like behavior.
Detailed Explanation
The behavior of light can be described through a theory known as wave-particle duality. This principle states that light acts as both a wave and a stream of particles (photons) under different circumstances. For instance, light can create patterns like waves in a ripple tank, yet it can also cause photoelectric effects by knocking electrons off materials when treated as discrete particles.
Examples & Analogies
To better understand this, consider how different sounds travel. If you think of singing as a wave in the air, that’s how sound travels as waves. However, when you hit a drum, you can imagine that energy is released in tiny packets like light, showing how it can act as both a continuous wave and as individual, separate impacts.
Key Concepts
-
Photon: A quantum of light with both wave-like and particle-like properties, and is massless.
-
Energy: Number of Joules represented as E = h·f, where h is Planck's constant.
-
Absorption: The process of capturing a photon, leading to electron excitation.
-
Emission: The release of a photon when an electron falls to a lower energy state.
-
Reflection: A surface phenomenon where light bounces off without being absorbed.
-
Refraction: The change in direction of light due to the change in medium.
-
Scattering: The deviation of light trajectories in various directions.
Examples & Applications
When a photon is absorbed by a silicon atom in a solar cell, it can generate an electron-hole pair, resulting in electricity.
In LEDs, when electrons recombine with holes, they emit photons, producing visible light.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Light is a wave, or a particle bright; energy flow is photon insight.
Stories
Once, in a glowing lab, photons danced across the ceiling, carrying energy and joy. They knew each color had a unique energy tune, and together they created a symphony of light!
Memory Tools
Remember 'AREEM' for Absorption, Reflection, Emission, and Scattering interactions with photons.
Acronyms
Use the acronym 'P.E.A.R.S.' - Photons Energy Absorption Reflection Scattering to recall key photon concepts.
Flash Cards
Glossary
- Photon
A quantum of light that exhibits both wave-like and particle-like properties.
- Energy
The capacity of a photon, given by E = h·f, where h is Planck's constant and f is frequency.
- Absorption
An interaction where a photon gets absorbed by an electron, raising it to a higher energy state.
- Emission
The process where electrons return to a lower energy state, releasing photons.
- Reflection
The bouncing of light off a surface.
- Refraction
The bending of light as it passes from one medium to another.
- Scattering
The random redirection of photons as they pass through a material.
Reference links
Supplementary resources to enhance your learning experience.
