CHANGE OF STATE
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Melting and Freezing
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're going to discuss two fundamental changes of state: melting and freezing. Can anyone tell me what happens when ice melts?

The ice turns into water!

That’s correct! When ice is heated to its melting point, it absorbs heat and changes to water without any temperature change. This heat is called latent heat. Can anyone explain what freezing is?

It’s when water turns back into ice!

Exactly! And during freezing, water releases heat. Remember, both ice and liquid water can coexist during the melting process until all ice is melted.

Why does the temperature stay constant during these changes?

Good question! The temperature stays constant during phase changes because all the absorbed or released energy is used for breaking intermolecular bonds rather than raising temperature.

To summarize: melting is the process of solid to liquid transition at a constant temperature, while freezing is the reverse process.
Vaporization
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let's talk about vaporization. Who can define what happens during the process of vaporization?

It’s when a liquid becomes a gas, right?

Exactly! Liquid water turns into vapor when heat is applied and reaches its boiling point. Like melting, vaporization occurs at a constant temperature until all liquid has been converted into gas.

What about boiling?

Boiling is a type of vaporization where the change occurs throughout the liquid. This definition is important as it highlights that vaporization can happen not just at the boiling point.

Does vaporization also have a heat energy term associated with it?

Yes, it does! This is termed the latent heat of vaporization. Energy is required to transition from liquid to gas without changing temperature.

To recap: Vaporization is the transition from liquid to gas, occurring at a constant temperature until all of the liquid has vaporized.
Phase Changes and Latent Heat
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s connect melting, freezing, and vaporization with latent heat. What is latent heat?

It's the energy needed for a phase change.

Correct! This energy is crucial for changes of state to occur at constant temperature. Can anyone give me examples of latent heat?

Like from ice to water or water to steam?

Exactly! Both those changes involve latent heat. Understanding this lets us comprehend why certain processes, like cooking or weather patterns, happen the way they do.

So, all those processes involve heat transfer?

Yes, they do! This is fundamental in thermal energy transfer. Let’s wrap up by reiterating that latent heat is the heat required for phase changes without temperature change.
Sublimation and Real-Life Applications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s touch on sublimation. What is it?

It's when a solid turns directly to gas without becoming a liquid!

Exactly right! An example is dry ice turning directly into vapor. How is this beneficial in real life?

It’s used in making fog effects for events and in refrigeration!

Well said! So, understanding changes of state is not only important academically but also in various applications—like your examples. Let's summarize what we've discussed today.

To recap: Sublimation is a direct change from solid to gas, and each phase change is a critical concept due to its real-world applications.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, we explore the changes of state in matter, such as melting, freezing, and vaporization, which occur due to heat exchange. We discuss the implications of temperature remaining constant during these changes and introduce the concept of latent heat, the energy required for these transitions without a temperature change. The significance of these concepts is emphasized through real-life applications and examples.
Detailed
Change of State
In this section, we delve into how matter transitions between its three primary states: solid, liquid, and gas. Understanding these changes is pivotal in thermodynamics and helps explain everyday phenomena.
Key Changes of State:
- Melting (Fusion): The transition from solid to liquid, which occurs at a material's melting point. For example, ice melts to become water when heat is supplied. During melting, the temperature remains constant until the entire solid has converted to a liquid, as energy is used to break molecular bonds.
- Freezing: The reverse process of melting, where a liquid turns into a solid. The freezing point is the temperature at which this transformation occurs.
- Vaporization: This refers to the change from liquid to gas, which also occurs at a specific temperature known as the boiling point. As a liquid heats up, it reaches a point where additional heat will convert the entire substance into gas without further temperature increases.
- Sublimation: Some materials like dry ice can transition directly from solid to gas, bypassing the liquid phase altogether. This process occurs under specific conditions of temperature and pressure.
Latent Heat:
An essential concept associated with changes of state is latent heat. This refers to the heat energy required for a phase change to occur at a constant temperature. For instance, when ice melts or water boils, heat is absorbed without changing the temperature of the substance.
Importance of Understanding Change of State:
- These principles are critical in various applications, including meteorology (understanding cloud formation), culinary science (cooking processes), and material science (manufacturing processes).
By grasping these concepts, we can better appreciate the physical world and the science underlying everyday occurrences.
Youtube Videos



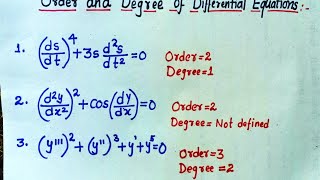
Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Change of State
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Matter normally exists in three states: solid, liquid and gas. A transition from one of these states to another is called a change of state. Two common changes of states are solid to liquid and liquid to gas (and, vice versa). These changes can occur when the exchange of heat takes place between the substance and its surroundings.
Detailed Explanation
Matter can exist in three forms: solid, liquid, and gas. A change from one of these states to another is termed a change of state. Common examples include the process of melting where a solid turns into a liquid and vaporization where a liquid becomes a gas. These transformations happen when heat is added or removed from the substance, leading to changes in molecular motion.
Examples & Analogies
Consider ice. When you heat ice cubes, they transform into water without immediate temperature change—this is melting. When boiling water, it will remain at 100 °C until all the water becomes steam, which demonstrates both heat absorption and a shift in state.
Melting and Freezing
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The change of state from solid to liquid is called melting or fusion and from liquid to solid is called freezing. It is observed that the temperature remains constant until the entire amount of the solid substance melts...
Detailed Explanation
During melting, the temperature of a substance remains constant despite continued heating. This happens because the heat energy goes into breaking the bonds between solid molecules. Conversely, during freezing, energy is released as the liquid turns back into solid form, and this transition also happens at a constant temperature until the entire liquid is frozen.
Examples & Analogies
When you heat ice in a pot, it reaches 0 °C and begins to melt. Even if you continue to heat, the temperature remains the same until all the ice turns into water. This process is similar to when water turns into ice—the temperature stays at 0 °C until all liquid water has become solid.
Vaporization and Boiling
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The change of state from liquid to vapor (or gas) is called vaporization. It is observed that the temperature remains constant until the entire amount of the liquid is converted into vapor...
Detailed Explanation
Similar to melting, the process of vaporization occurs at a constant temperature called the boiling point. When water reaches this point, it starts to turn into steam without any increase in temperature. This means all the energy goes into changing the state of the water from liquid to gas until there is no liquid left.
Examples & Analogies
Think of boiling water on the stove. You notice that it heats to 100 °C, but even if you keep heating, the temperature stays the same until all the water evaporates. The heat energy at this stage is used to change water into steam instead of raising the temperature.
Triple Point and Phase Diagrams
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A graph between the temperature T and the Pressure P of the substance is called a phase diagram. Such a phase diagram divides the P – T plane into a solid-region, the vapour-region and the liquid-region.
Detailed Explanation
A phase diagram visually represents how phases of a substance (solid, liquid, gas) exist together at different temperatures and pressures. For instance, the triple point is the specific condition where all three states coexist in equilibrium, which offers insights into substance behaviors under various conditions.
Examples & Analogies
Visualize a sealed can of soda. In a certain range of temperature and pressure, you can find the carbon dioxide in three forms: gas bubbles, dissolved CO₂ liquid, and solid ice. A phase diagram would help illustrate the conditions where all these forms exist together. This is similar to how ice, water, and steam coexist in a controlled environment at specific conditions.
Latent Heat
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The amount of heat per unit mass transferred during change of state of the substance is called latent heat of the substance for the process.
Detailed Explanation
Latent heat refers to the energy required to change a substance from one state to another without changing its temperature. For example, when ice melts into water, it absorbs heat (latent heat of fusion) without raising its temperature until all the ice has melted. Similarly, liquid water absorbs latent heat during vaporization without temperature change until it fully converts to steam.
Examples & Analogies
When cooking, if you were to add heat to a pot of ice, you'd observe the ice absorbs heat without the temperature increasing until all has melted. This concept explains why some substances can greatly cool the surrounding air when they melt—such as ice in a drink—absorbing significant latent heat in the process.
Key Concepts
-
Melting: The transition from solid to liquid.
-
Freezing: The process of liquid turning to solid.
-
Vaporization: Transition from liquid to gas.
-
Latent Heat: Energy during phase changes without temperature change.
-
Sublimation: Solid to gas transition.
Examples & Applications
Ice melting into water when heated.
Water boiling into steam when heated to 100°C.
Dry ice sublimating into carbon dioxide gas.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When ice turns to drink, it melts with a blink.
Stories
Imagine a block of ice on a warm day. It starts to melt slowly. A child waits eagerly with a cup, and as the ice melts, it fills the cup with cool water.
Memory Tools
Melt = solid to liquid; Boil = liquid to gas - Remember: 'Melt the Ice, Boil the Tea!'
Acronyms
L.E.M. - Latent Energy for Melting (or phase changes).
Flash Cards
Glossary
- Melting
The transition from solid to liquid at a specific temperature.
- Freezing
The transition from liquid to solid at a specific temperature.
- Vaporization
The process of a liquid turning into a gas at its boiling point.
- Latent Heat
The heat required to change the phase of a material without changing its temperature.
- Sublimation
The process of a solid turning directly into gas without going through the liquid phase.
Reference links
Supplementary resources to enhance your learning experience.
