NEWTON’S LAW OF COOLING
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Newton's Law of Cooling
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're discussing Newton's Law of Cooling. Let's start with a question: what happens when you leave a hot cup of coffee on the table?

It cools down over time.

Exactly! And why does that happen?

Because it loses heat to the surrounding air.

Good point! This process is what Newton's Law of Cooling describes. It states that the rate at which the heat is lost is proportional to the temperature difference between the coffee and the environment.

So if the room is really cold, will it cool down faster?

Exactly! The greater the temperature difference, the faster it cools. Remember this with the acronym 'COLD'—Cooling is Optimal with a larger Difference.

That's a useful tip!

Let’s recap: Newton's Law helps us understand heat loss, which is vital in many real-life applications. Next, we’ll look at how we can mathematically express this concept.
Mathematical Expression of Cooling
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s get into the formula behind Newton's Law of Cooling. It’s expressed as \(-\frac{dQ}{dt} = k(T_2 - T_1)\). What do you think each term represents?

\(-\frac{dQ}{dt}\) is the rate of heat loss.

Correct! And what about \(T_2\) and \(T_1\)?

\(T_2\) is the temperature of the object and \(T_1\) is the temperature of the surroundings.

Exactly! The constant \(k\) depends on factors like the surface area of the object. This formula allows us to predict how long it will take for an object to cool down under specific conditions. Remember the term 'TROC'—Temperature Rate Of Change!

So, we can use this to predict cooling times in real-life situations?

Absolutely! Let's do some calculations together, bringing these concepts into practice.
Applications and Real-Life Examples
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we’ve covered the formula, how can we apply Newton’s Law of Cooling in real life? Any ideas?

Maybe in cooking, to know how long food needs to cool down?

Exactly! Understanding how quickly food cools can help in food safety and recipe timing. What else?

It might also apply to experiments and how quickly a solution might cool.

Spot on! In labs, temperatures need to be controlled precisely. Also, think about cooling a car engine after it has been running hot. We're constantly using Newton’s Cooling Law, often without even realizing it.

That's really interesting! It makes sense why temperature differences matter.

Let’s summarize: Newton's Law of Cooling is a key principle not just in physics, but in everyday life scenarios. Keep looking for real applications!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Newton's Law of Cooling provides a quantitative way to understand how and why hot objects cool down over time. It states that the rate of heat loss of a body is directly proportional to the temperature difference between the body and its environment, applicable for small temperature differences. The section also includes experiments illustrating this cooling law.
Detailed
Detailed Summary
Newton's Law of Cooling is fundamentally important when discussing heat transfer between a body and its surroundings. The law states that the rate of loss of heat by a body is directly proportional to the difference in temperature between the object and its surrounding medium, as long as this difference is small. This simple yet powerful concept was first derived by Sir Isaac Newton and provides a foundational perspective in thermodynamics.
Key Concepts:
- Cooling Experiment: An experiment with a calorimeter is described where heated water cools over time, showcasing how the temperature difference influences the cooling rate.
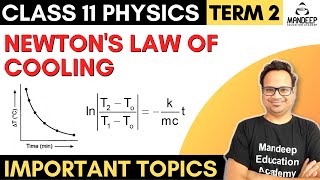
- Mathematical Representation: The law is mathematically expressed as:
\[
\frac{-dQ}{dt} = k(T_2 - T_1)
\]
where \( T_2 \) is the temperature of the body, \( T_1 \) is the temperature of the surroundings, and \( k \) is a constant related to the object's surface area and nature. - Integration and Practical Application: By applying calculus, we can derive equations that allow for predictions of cooling times under certain conditions, which is useful in various practical scenarios such as determining the optimal cooling times in culinary settings or for scientific measurements.
Overall, understanding Newton's Law of Cooling has significant implications for thermodynamics, scientific studies, and practical applications.
Youtube Videos




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Cooling
Chapter 1 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
We all know that hot water or milk when left on a table begins to cool, gradually. Ultimately it attains the temperature of the surroundings.
Detailed Explanation
When you leave a hot liquid like water or milk out in a room, it gradually loses heat to the surrounding air. This continues until the temperature of the liquid matches the temperature of its surroundings. This process illustrates the fundamental concept of heat transfer, where heat flows from a hotter object to a cooler one.
Examples & Analogies
Imagine taking a warm cup of coffee from the kitchen and setting it on the table. The coffee, which is hotter than the room temperature, starts to lose its warmth. You can feel this if you touch the cup after a while; it will no longer feel hot but warm or even cool, showing heat loss.
Setting Up the Experiment
Chapter 2 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
To study how slow or fast a given body can cool on exchanging heat with its surroundings, let us perform the following activity. Take some water, say 300 mL, in a calorimeter with a stirrer and cover it with a two-holed lid. Fix the stirrer through one hole and fix a thermometer through another hole in the lid and make sure that the bulb of thermometer is immersed in the water. Note the reading of the thermometer. This reading T1 is the temperature of the surroundings.
Detailed Explanation
In this experiment, you will set up a calorimeter containing hot water to measure its cooling over time. The thermometer monitors the water’s temperature while stirring helps to maintain a uniform temperature throughout the water. The initial thermometer reading (T1) indicates the temperature of the surroundings, which will be important in calculating the rate of heat loss.
Examples & Analogies
Think of this set-up as putting a hot soup in a thermos while checking its temperature at intervals. Just like the thermometer measures the soup’s heat loss over time, the experiment measures how the water in the calorimeter cools when exposed to the air around it.
Observing and Recording the Cooling Process
Chapter 3 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Heat the water kept in the calorimeter till it attains a temperature, say 40 °C above room temperature (i.e., temperature of the surroundings). Then, stop heating the water by removing the heat source. Start the stop-watch and note the reading of the thermometer after a fixed interval of time, say after every one minute of stirring gently with the stirrer.
Detailed Explanation
After heating the water, it is crucial to stop the heating source so that the cooling process begins without any additional heat being added. You will observe the temperature decrease as time progresses, which you record at regular intervals. This helps in determining how quickly the temperature drops over time.
Examples & Analogies
Picture a race timer at a sports event: once the starting signal is given (stopping the heat), the runners (water temperature) begin to slow down as they head to the finish line (room temperature). Each minute you check the temperature is like checking how far along the runners are in the race.
Understanding the Graph of Cooling
Chapter 4 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Continue to note the temperature (T2) of water till it attains a temperature about 5 °C above that of the surroundings. Then, plot a graph by taking each value of temperature ∆T = T2 – T1 along y-axis and the corresponding value of t along x-axis.
Detailed Explanation
After recording temperatures until the water cools down near the surrounding temperature, plotting these results on a graph helps visualize the cooling trend. The y-axis represents the temperature difference (∆T) between the water and the surroundings, while the x-axis denotes time. This graphical representation can demonstrate how the rate of cooling changes over time.
Examples & Analogies
Creating this graph is much like mapping a journey on a road trip. The y-axis shows how far you’ve traveled from hot temperatures to cool ones as you move along the x-axis (time) – it helps in understanding how quickly you're reaching your destination of equilibrium with the surroundings.
Newton's Law of Cooling
Chapter 5 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The above activity shows that a hot body loses heat to its surroundings in the form of heat radiation. The rate of loss of heat depends on the difference in temperature between the body and its surroundings. Newton was the first to study, in a systematic manner, the relation between the heat lost by a body in a given enclosure and its temperature.
Detailed Explanation
Newton's Law of Cooling states that the rate at which a body cools is directly proportional to the temperature difference between the body and the environment. This means that the greater the temperature difference, the faster the rate of cooling. This relationship helps us understand cooling processes in various applications, such as cooling drinks or heat loss in buildings.
Examples & Analogies
Think of it like a warm pie on a cold winter day. If you leave it outside, it cools down faster when it’s very hot compared to when it’s just slightly warm. It's the difference in temperature that determines how quickly it cools, not just the fact that it’s warm.
Mathematical Representation
Chapter 6 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
According to Newton’s law of cooling, the rate of loss of heat, –dQ/dt of the body is directly proportional to the difference of temperature ∆T = (T2–T1) of the body and the surroundings. The law holds good only for small difference of temperature.
Detailed Explanation
Mathematically, this relationship is expressed as -dQ/dt = k(T2 - T1), where dQ is the heat lost, dt is the time taken, k is a constant, T2 is the temperature of the body, and T1 is the surrounding temperature. This equation quantifies cooling in practical scenarios, as it shows how changes in temperature impact the rate of heat loss.
Examples & Analogies
Consider how a car engine cools after being turned off. At first, the heat difference between the hot engine and cooler air is large, resulting in rapid cooling. As it cools down, the difference decreases, and so does the rate of cooling. You can visualize this with the equation, helping to predict how quickly an engine will get back to ambient temperature.
Conclusion and Applications
Chapter 7 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For small temperature differences, the rate of cooling, due to conduction, convection, and radiation combined, is proportional to the difference in temperature.
Detailed Explanation
In conclusion, Newton's Law of Cooling helps us understand how various factors like conduction, convection, and radiation contribute to the cooling process. This understanding is applicable in everyday situations, ranging from engineering, cooking, and even weather phenomena. For practical usage, it assists engineers in designing systems to maintain or alter temperature effectively.
Examples & Analogies
Think of thermos bottles that keep drinks hot or cold. They employ concepts from Newton's law of cooling by minimizing heat exchange with the surroundings through insulation. Understanding how heat transfer works allows us to create better products for our daily lives.
Key Concepts
-
Cooling Experiment: An experiment with a calorimeter is described where heated water cools over time, showcasing how the temperature difference influences the cooling rate.
-
Mathematical Representation: The law is mathematically expressed as:
-
\[
-
\frac{-dQ}{dt} = k(T_2 - T_1)
-
\]
-
where \( T_2 \) is the temperature of the body, \( T_1 \) is the temperature of the surroundings, and \( k \) is a constant related to the object's surface area and nature.
-
Integration and Practical Application: By applying calculus, we can derive equations that allow for predictions of cooling times under certain conditions, which is useful in various practical scenarios such as determining the optimal cooling times in culinary settings or for scientific measurements.
-
Overall, understanding Newton's Law of Cooling has significant implications for thermodynamics, scientific studies, and practical applications.
Examples & Applications
A cup of coffee left on the table cools down gradually due to heat loss to the surrounding air.
In cooking, understanding the cooling rate can prevent food from becoming unsafe to eat.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When it's hot, and starts to cool, the higher the temp, the faster the rule!
Stories
Imagine leaving a hot pizza on the counter. At first, it's steaming hot, gradually becoming warm, then cool. This illustrates Newton's Law where the cooling rate slows as the pizza approaches room temperature.
Memory Tools
COLD: Cooling is Optimal with a larger Difference.
Acronyms
TROC
Temperature Rate Of Change
highlighting how temperature drives cooling.
Flash Cards
Glossary
- Newton's Law of Cooling
A principle that states the rate of loss of heat is directly proportional to the temperature difference between an object and its surroundings.
- Heat Loss
The transfer of thermal energy from an object to its environment when they have different temperatures.
- Temperature Difference
The difference in temperature between an object and its surroundings, which drives heat transfer.
- Constant (k)
A constant of proportionality in the cooling equation that depends on the characteristics of the object and its environment.
Reference links
Supplementary resources to enhance your learning experience.
