Hydration Reactions of Mineral Admixtures
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Pozzolanic Reaction Mechanism
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are going to discuss the pozzolanic reaction mechanism. Can anyone tell me what happens during this reaction?

Isn’t it when mineral admixtures react with calcium hydroxide to form C-S-H?

Exactly! The equation for the reaction is SiO₂ + Ca(OH)₂ + H₂O → C-S-H. Through this process, we create more of the strength-giving compound in concrete. What does C-S-H do to the structure?

It makes the concrete stronger and less permeable!

Correct! By creating more C-S-H, the pore structure is refined, which leads to lower permeability. This is essential for the durability of concrete.

So, does that mean more pozzolans lead to stronger concrete?

Yes! But the effectiveness depends on the type of pozzolan and the conditions of use. Remember the acronym 'S-P-D' which stands for Strength, Permeability, and Durability when discussing the benefits.
Impact on Microstructure
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's dive into how these reactions impact the microstructure of concrete. Who can summarize some effects?

The reactions lead to a refined pore structure and increase the volume of C-S-H gel!

Great point! The increase in C-S-H reduces Ca(OH)₂ crystals. What does this mean for the concrete?

It minimizes leaching and efflorescence, right? That’s important for maintaining structural integrity over time.

Exactly! And a denser microstructure ultimately improves strength and long-term durability. Let's remember this with the mnemonic 'DLS' - Density, Leaching, Strength.

That's easy to remember!
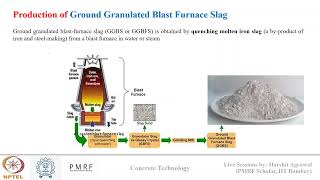
Hydraulic Reaction of GGBS
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Moving on, let's discuss how GGBS undergoes hydration. Does anyone know how this differs from pozzolanic reactions?

GGBS can react like cement when it’s in the presence of water and alkaline activators.

Correct! This reaction forms both C-S-H and calcium aluminate hydrates (C-A-H). Why is this important?

It contributes to the overall strength, similar to traditional cement!

Exactly. Just keep in mind that GGBS generally leads to slower early strength gain but improved long-term benefits—‘SLB’ can help you remember: Slower Early, Better long-term.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Mineral admixtures undergo pozzolanic and hydraulic reactions during hydration, creating calcium silicate hydrate (C-S-H) which enhances the strength and durability of concrete. The section covers the mechanisms involved and their impact on the microstructure and properties of concrete.
Detailed
In this section, we explore the hydration reactions of mineral admixtures, primarily focusing on the pozzolanic reaction mechanisms where materials react with calcium hydroxide (Ca(OH)₂) produced during cement hydration. This process generates additional calcium silicate hydrate (C-S-H), a key component that contributes to the strength of concrete.
Furthermore, different mineral admixtures have unique impacts on concrete's microstructure:
- Refined Pore Structure: The additional C-S-H leads to a denser and less permeable structure, enhancing durability.
- Reduction of Ca(OH)₂ Crystals: This reduces leaching and efflorescence in concrete, promoting structural integrity over time.
- Hydraulic Reactions: Particularly noted with Ground Granulated Blast Furnace Slag (GGBS), which reacts similarly to Portland cement in the presence of water and alkaline activators, contributing further to the strength of the mix. Understanding these hydration reactions is crucial for optimizing the performance of concrete containing mineral admixtures.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Pozzolanic Reaction Mechanism
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Most mineral admixtures undergo a secondary hydration process known as pozzolanic reaction, where they react with calcium hydroxide (Ca(OH)₂)—a by-product of cement hydration—to form additional calcium silicate hydrate (C-S-H), the main strength-giving compound in concrete.
Basic Reaction (Simplified):
SiO₂ + Ca(OH)₂ + H₂O → C-S-H
Detailed Explanation
In this chunk, we explore the pozzolanic reaction, which is a vital chemical reaction that occurs when mineral admixtures like fly ash or silica fume react with calcium hydroxide from hydrated cement. This reaction produces calcium silicate hydrate (C-S-H), which is the primary compound that contributes to the strength and durability of concrete. The equation presented illustrates how silicon dioxide (SiO₂) from the mineral admixture, water (H₂O), and calcium hydroxide (Ca(OH)₂) combine to create C-S-H. This process increases the overall strength of the concrete mix.
Examples & Analogies
You can think of this reaction like cooking. Just as you need certain ingredients to make a dish, such as flour, water, and yeast to make bread, the pozzolanic materials need the right conditions (like the presence of Ca(OH)₂ and water) to react and form a strong concrete structure.
Impact on Microstructure
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Refinement of pore structure: Leads to lower permeability and higher density.
- Reduction in Ca(OH)₂ crystals: Minimizes leaching and efflorescence.
- Increased volume of C-S-H gel: Improves long-term strength and durability.
Detailed Explanation
This chunk outlines the beneficial effects of the pozzolanic reaction on the microstructure of concrete. First, by refining the pore structure, the reaction reduces the size and number of pores, making the concrete denser and less permeable. This means that water and harmful substances can't easily seep through the concrete, enhancing its durability. Additionally, as the pozzolanic reaction reduces the crystals of calcium hydroxide, it mitigates issues like leaching (where materials are washed away) and efflorescence (the formation of white, powdery deposits on the surface). Finally, the increase in the volume of C-S-H gel strengthens the concrete over time, ensuring it remains robust and reliable under various conditions.
Examples & Analogies
Imagine a sponge. A sponge with small, tightly packed pores can hold more liquid and is less likely to leak than one with large, scattered holes. Similarly, the refinement of pore structure in concrete helps it resist water penetration and makes it stronger over time.
Hydraulic Reaction (GGBS)
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In the presence of water and alkaline activators (like calcium hydroxide from OPC), GGBS undergoes hydration similar to Portland cement, forming:
GGBS + H₂O → C-S-H + C-A-H (calcium aluminate hydrates)
Detailed Explanation
GGBS (Ground Granulated Blast Furnace Slag) performs a different reaction in the presence of water and alkaline activators like calcium hydroxide, resulting in hydration akin to that of traditional Portland cement. Here, not only does it produce C-S-H (about which we have already learned) but also calcium aluminate hydrates (C-A-H). This dual reaction further contributes to the strength and durability of concrete, making GGBS an effective mineral admixture in construction.
Examples & Analogies
Think of this as a mix of various ingredients in a recipe. When you combine necessary activators (like the water and alkaline environment) with GGBS, it is like adding yeast to dough; it triggers the mix to rise and gain structure, making it stronger and more resilient.
Key Concepts
-
Pozzolanic Reaction: This increases the amount of C-S-H which improves strength and durability.
-
Hydraulic Reaction with GGBS: GGBS reacts similarly to cement in the presence of alkaline activators, enhancing concrete properties.
-
Microstructure Impact: Enhanced microstructure leads to lower permeability and higher density.
Examples & Applications
An example of a pozzolanic reaction is the use of silica fume in concrete, which significantly enhances the strength due to the formation of C-S-H.
When GGBS is added to a concrete mix, it reacts with water and helps reduce heat of hydration while contributing to long-term strength.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
C-S-H from SiO₂, with Ca(OH)₂ and water too; a stronger concrete is our goal, as we refine that pore control!
Stories
Imagine the concrete as a building, and C-S-H as the bricks that make it stronger and more resilient against the weather over time.
Memory Tools
‘S-P-D’ for Strength, Permeability, and Durability; remember what pozzolanic actions do!
Acronyms
‘SLB’ for Slower Early, Better long-term applied to GGBS effects.
Flash Cards
Glossary
- CSH
Calcium Silicate Hydrate; the primary strength-giving component formed during hydration reactions in concrete.
- Pozzolanic Reaction
A secondary hydration process where silica in mineral admixtures reacts with calcium hydroxide to create additional C-S-H.
- Hydraulic Reaction
A reaction where materials like GGBS hydrate in the presence of water, resulting in cementitious compounds.
- Calcium Hydroxide
A by-product of cement hydration, typically contributing to concrete's alkalinity and strength development.
- Microstructure
The fine structural features of concrete that influence its properties, such as permeability and strength.
Reference links
Supplementary resources to enhance your learning experience.
