Classification of Materials by Conductivity
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Conductors and Their Characteristics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we'll discuss conductors, which are essential for the flow of electricity in circuits. Can anyone tell me which materials commonly act as conductors?

Copper and silver are good conductors!

Exactly! They allow for very high electrical conductivity. Conductors have overlapping energy bands, which means electrons can move freely. What do you think happens if we increase the temperature of a conductor?

Maybe its conductivity would change a little, but it's still a conductor?

Good guess! However, unlike semiconductors, their conductivity remains relatively constant despite temperature changes. That's a key difference. So remember: Conductors = Very High Conductivity, Overlapping Bands.
Understanding Semiconductors
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's turn our attention to semiconductors. Who can define what a semiconductor is?

A semiconductor has moderate conductivity and it can conduct electricity under certain conditions!

Great! Specifically, semiconductors usually have an energy band gap of about 1 eV. What do you think this means for materials like silicon or germanium?

It means that they can conduct electricity better when they're heated or doped!

Exactly! That's why they're used in devices like transistors and diodes. It's all about manipulating that band gap for efficient conductivity. Remember: Semiconductors = Moderate Conductivity, ~1 eV.
Role of Insulators in Electronics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let's look at insulators. Who can tell me about their properties?

I know insulators have very low conductivity and a large band gap!

That's right! Insulators like glass and rubber have energy band gaps greater than 5 eV, making them poor conductors. Can anyone think of a real-world application for insulators?

They keep electrical wires safe and prevent electric shocks!

Exactly! Insulators are crucial for safety in electrical systems. So just remember, Insulators = Very Low Conductivity, >5 eV band gap.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The classification of materials by conductivity involves categorizing materials into conductors, semiconductors, and insulators based on their electrical conductivity and energy band gaps. This section emphasizes examples and the significance of understanding these classifications in semiconductor physics.
Detailed
Classification of Materials by Conductivity
The section delves into the classification of materials based on their electrical conductivity, which is a crucial aspect in the field of semiconductor physics. Materials can be categorized into three primary types:
1. Conductors
- Electrical Conductivity: Very High
- Energy Band Gap (Eg): Overlapping bands
- Example Materials: Copper, Silver
Conductors are materials that allow electric current to flow freely due to their very high electrical conductivity. The energy bands in conductors overlap, meaning that electrons can move without significant energy barriers.
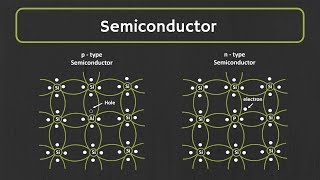
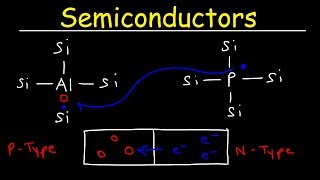
2. Semiconductors
- Electrical Conductivity: Moderate
- Energy Band Gap (Eg): Approximately 1 eV
- Example Materials: Silicon, Germanium
Semiconductors have moderate conductivity and are unique due to their ability to conduct electricity under certain conditions (e.g., temperature change or doping with impurities). The 1 eV band gap of semiconductors is crucial for their function in electronic devices. They are the backbone of modern electronics, leading to the development of various devices such as diodes and transistors.
3. Insulators
- Electrical Conductivity: Very Low
- Energy Band Gap (Eg): Greater than 5 eV
- Example Materials: Glass, Rubber
Insulators are materials that do not conduct electricity well, primarily due to their very low conductivity and significant energy band gap, which prevents electrons from jumping from the valence band to the conduction band. Understanding these classifications helps in utilizing materials effectively in designing electronic components.
Youtube Videos


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Overview of Material Conductivity Types
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
| Type | Electrical Conductivity | Energy Band Gap (Eg) | Example Materials |
|---|---|---|---|
| Conductors | Very High | Overlapping bands | Copper, Silver |
| Semiconductor | Moderate | ~1 eV | Silicon, Germanium |
| Insulators | Very Low | > 5 eV | Glass, Rubber |
Detailed Explanation
This chunk provides a classification of materials based on their electrical conductivity, which is categorized into three main types: conductors, semiconductors, and insulators.
- Conductors have very high electrical conductivity because their energy bands overlap, allowing electrons to move freely. Examples include copper and silver.
- Semiconductors have moderate conductivity, typically around 1 electron volt (eV), and are key in electronic devices. Silicon and germanium are common semiconductors.
- Insulators have very low conductivity (greater than 5 eV energy gap), which prevents the flow of electric current. Glass and rubber fall into this category.
Examples & Analogies
Think of conductors as highways with no traffic (like copper), allowing electrons to zip by without obstruction. Semiconductors are like toll roads (like silicon), where some control exists over who can pass and how easily. Insulators are like closed roads (like rubber), where you can’t pass at all.
Detailed Comparison of Conductivity Types
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
| Type | Electrical Conductivity | Energy Band Gap (Eg) | Example Materials |
|---|---|---|---|
| Conductors | Very High | Overlapping bands | Copper, Silver |
| Semiconductors | Moderate | ~1 eV | Silicon, Germanium |
| Insulators | Very Low | > 5 eV | Glass, Rubber |
Detailed Explanation
This chunk further emphasizes the differences in conductivity and energy band gaps for the three categories:
- Conductors: High conductivity is due to the overlapping energy bands, which allow electrons to flow easily.
- Semiconductors: They have a moderate conductivity due to their energy band gap of about 1 eV, which allows some electrons to jump to the conduction band under certain conditions, like temperature changes.
- Insulators: The high energy band gap (over 5 eV) means that not enough energy is available for electrons to move freely, making them unable to conduct electricity under normal circumstances.
Examples & Analogies
Imagine a park with different paths: wide, clear paths (conductors) allow people to roam freely; narrower paths with some obstacles (semiconductors) may limit movement, but allow some access when needed; while full fences (insulators) keep everyone out completely.
Key Concepts
-
Conductors: Very high conductivity and overlapping energy bands.
-
Semiconductors: Moderate conductivity with ~1 eV energy band gap.
-
Insulators: Very low conductivity with energy band gaps > 5 eV.
Examples & Applications
Copper and silver are classic examples of conductors used in electrical wiring due to their excellent conductivity.
Silicon is widely used in electronic components like transistors due to its semiconductor properties.
Rubber is commonly used as insulation around wires to prevent electrical conductivity.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Conductors flow like rivers bright, / Semis spark with a thermal light. / Insulators stand firm with a might, / Preventing shocks with all their might.
Stories
Once upon a time, there were three villages: Conductor, Semiconductor, and Insulator. Conductor was known for its swift rivers, flowing freely. Semiconductor was unique, gaining power when it warmed up, but sometimes it struggled. Insulator was sturdy, always protecting others from shocks.
Memory Tools
C (Conductor - Very High), S (Semiconductor - Moderate), I (Insulator - Very Low). 'CSI' can help you remember their conductivity levels.
Acronyms
C-S-I
Conductors (C) have high conductivity
Semiconductors (S) have moderate conductivity
Insulators (I) have low conductivity.
Flash Cards
Glossary
- Conductors
Materials with very high electrical conductivity, such as copper and silver, where the energy bands overlap.
- Semiconductors
Materials that have moderate conductivity and an energy band gap of about 1 eV, such as silicon and germanium.
- Insulators
Materials with very low conductivity and a significant energy band gap greater than 5 eV, such as glass and rubber.
- Energy Band Gap (Eg)
The energy difference between the valence band and conduction band in a material, influencing its conductivity.
Reference links
Supplementary resources to enhance your learning experience.
