Overview of Semiconductor Physics
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Semiconductors
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome, class! Today we're discussing semiconductors. Can anyone tell me what semiconductors are?

Are they materials that conduct electricity?

Exactly! Semiconductors are materials that have electrical conductivity between conductors and insulators. They are crucial in modern electronics.

What are some examples of semiconductors?

Great question! Common examples include Silicon and Germanium. Remember, S and G for Semiconductors!

Why are they important?

They form the basis of electronic components like diodes and transistors. Understanding their properties helps us create advanced technologies.

What is energy band theory?

Energy band theory describes how electrons are organized in materials, which we'll explore further. Let's take it step by step!

To summarize: Semiconductors are key materials between conductors and insulators, with Silicon and Germanium being common examples.
Energy Band Theory
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss energy band theory. Who knows what the valence band and conduction band are?

Is the valence band where electrons are held?

Exactly right! The valence band contains electrons that are bound to atoms. Whereas the conduction band is where electrons can move freely, allowing for conduction of electricity.

What does the forbidden energy gap mean?

Good observation! The forbidden energy gap, or Eg, is the energy difference between the valence band and conduction band. In semiconductors, this gap is around 1 eV, allowing some electrons to jump when they gain energy.

What happens to semiconductors at low temperatures?

At 0 K, pure semiconductors behave like insulators because there are no free charge carriers.

To conclude this session, remember that the behavior of semiconductors is governed by energy band theory, with valence and conduction bands separated by a forbidden energy gap.
Classifications of Materials by Conductivity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let’s categorize materials based on conductivity. Can anyone name the types?

Conductors, semiconductors, and insulators?

Spot on! Conductors have very high conductivity, while insulators have very low conductivity. Semiconductors fall in between.

What are some examples of each type?

Conductors include Copper and Silver, semiconductors are Silicon and Germanium, and insulators could be Glass and Rubber!

What makes semiconductors unique?

Semiconductors can be modified by doping, which allows us to control their conductivity. We'll explore that in more detail later!

To wrap up, remember the key classifications: Conductors have high conductivity, Insulators have low, and Semiconductors sit in the middle!
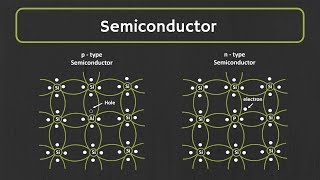
Intrinsic and Extrinsic Semiconductors
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s explore intrinsic and extrinsic semiconductors. Who can explain what intrinsic semiconductors are?

Are they pure semiconductors without impurities?

That’s right! They solely rely on thermally generated electron-hole pairs. Now, what about extrinsic semiconductors?

Those are semiconductors that are doped with impurities, right?

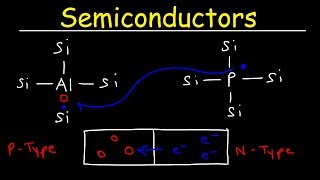
Correct! Doping introduces additional charge carriers, and we have two types: n-type, which has extra electrons, and p-type, which has extra holes.

Can you remind us what n-type and p-type mean?

Sure! n-type semiconductors have electrons as majority carriers, while p-type semiconductors have holes as majority carriers. Phosphorus is a common dopant for n-type, and Boron for p-type.

To summarize, intrinsic semiconductors are pure, while extrinsic ones are doped to enhance their conductivity.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section introduces semiconductors, which are materials vital for electronic devices. Key concepts include energy band theory, the classification of materials based on conductivity, and the transition between intrinsic and extrinsic semiconductors.
Detailed
Overview of Semiconductor Physics
Semiconductors are materials that exhibit electrical conductivity levels between those of conductors (like metals) and insulators (like glass). They are essential in the field of electronics, forming the backbone of many devices such as diodes, transistors, and integrated circuits. Common semiconductor materials include Silicon (Si) and Germanium (Ge). The behavior of semiconductors is explained through energy band theory, which involves concepts such as the valence band, conduction band, and forbidden energy gap (Eg).
The section also categorizes materials based on their conductivity:
- Conductors: Have very high conductivity, with overlapping energy bands (e.g., Copper, Silver).
- Semiconductors: Have moderate conductivity with energy band gaps around 1 eV (e.g., Silicon, Germanium).
- Insulators: Have very low conductivity with energy gaps greater than 5 eV (e.g., Glass, Rubber).
Understanding these foundational concepts is critical for delving deeper into topics like intrinsic and extrinsic semiconductors, energy band diagrams, and the principles of drift and diffusion currents.
Youtube Videos


Audio Book
Dive deep into the subject with an immersive audiobook experience.
What are Semiconductors?
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Semiconductors are materials with electrical conductivity between that of conductors and insulators. They are the foundation of modern electronics, used in devices like diodes, transistors, and integrated circuits.
Detailed Explanation
Semiconductors are unique materials that fall in between good conductors (like metals) and insulators (like rubber). This means they can conduct electricity, but not as well as metals. Semiconductors are crucial in electronics because they form the building blocks of many important devices. For instance, a diode allows current to flow in only one direction, while a transistor can amplify signals, making it vital in all electronic devices.
Examples & Analogies
Think of semiconductors like a gate that can be opened or closed. When it's closed, electricity can't flow (like an insulator), but when it's opened, electricity can flow (like a conductor). This ability to control the flow of electricity is what makes semiconductors incredibly useful in gadgets like smartphones and computers.
Common Semiconductors
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Common semiconductors: Silicon (Si) and Germanium (Ge)
Detailed Explanation
Silicon (Si) and Germanium (Ge) are the two most widely used materials in semiconductor manufacturing. Silicon is particularly popular because of its abundance and excellent electrical properties. Germanium, while also a semiconductor, is less commonly used today but was one of the first materials utilized in electronics. Understanding these materials is key to grasping how electronic devices function.
Examples & Analogies
Imagine Silicon and Germanium as the primary chefs in a kitchen. Silicon is the head chef who is favored because they can whip up dishes quickly and efficiently, while Germanium is a skilled sous-chef who used to be in charge but is now used for special gourmet dishes only. Silicon’s efficiency and availability make it the go-to choice for most electronic dishes!
Energy Band Theory
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Energy band theory explains their behavior using valence band, conduction band, and forbidden energy gap (Eg).
Detailed Explanation
Energy band theory provides a model to understand how semiconductors work. Electrons in a semiconductor can exist in two main energy bands: the valence band, where they are bound to atoms, and the conduction band, where they are free to move and conduct electricity. The gap between these bands is called the forbidden energy gap (Eg). This gap is crucial because it determines how easily electrons can jump from the valence to the conduction band, allowing for electrical conductivity. Depending on temperature and impurities, this gap changes the material's conductivity characteristics significantly.
Examples & Analogies
Think of the valence band as a lower floor in a building and the conduction band as an upper floor. The elevator (forbidden energy gap) represents the energy needed for the electrons (people) to move from the lower to the upper floor. If the elevator is broken (large Eg), no one can reach the upper floor (no conductivity). However, if you increase the elevator's power (which can be seen as increasing temperature), more people can reach the upper floor, and conductivity increases.
Key Concepts
-
Semiconductors bridge conductors and insulators, crucial for electronic devices.
-
Energy band theory helps explain semiconductor behavior.
-
Intrinsic semiconductors are pure, extrinsic are doped with impurities.
-
Conductivity is affected by temperature and the presence of impurities.
Examples & Applications
Silicon and Germanium are common examples of semiconductors, used extensively in electronics for diodes, transistors, and integrated circuits.
Copper and Silver are examples of conductors used in electrical wiring, while Glass and Rubber are used as insulators.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Silicon and Germ, in devices they stem, semiconductors shine, making tech so fine.
Stories
Once upon a time in the land of silicon, the tiny electrons danced between bands, jumping the gap to create magic in devices.
Memory Tools
Remember: 'S-G for Semiconductors' (Silicon and Germanium).
Acronyms
D-I-E
Doping affects Intrinsic and Extrinsic semiconductors.
Flash Cards
Glossary
- Semiconductor
Materials that have electrical conductivity between conductors and insulators.
- Energy band theory
A theory that explains the behavior of electrons in solid materials, particularly regarding their valence and conduction bands.
- Valence band
The energy band that contains electrons bound to atoms.
- Conduction band
The energy band where electrons can move freely, conducting electricity.
- Forbidden energy gap (Eg)
The energy difference between the valence band and conduction band.
- Intrinsic semiconductor
A pure semiconductor without any impurities.
- Extrinsic semiconductor
A semiconductor that has been doped with impurities to control its electrical properties.
Reference links
Supplementary resources to enhance your learning experience.
