Atomic Structure of Semiconductors
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Valence Electrons in Semiconductors
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're focusing on Silicon and Germanium, which are key semiconductors. Can anyone tell me how many valence electrons they have?

Is it four valence electrons?

That's correct! Each has four valence electrons. This is crucial for forming covalent bonds. Can anyone explain what covalent bonds are?

Covalent bonds are when atoms share electrons to fill their outer shells.

Exactly! This sharing of electrons helps stabilize the atomic structure in semiconductors. Remember, 'Four for the core!' helps us keep in mind that both Si and Ge share four valence electrons!
Behavior of Semiconductors at Absolute Zero
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

At absolute zero, what do you think happens to semiconductors?

I think they act like insulators since electrons are bound tightly?

Yes! At 0 K, all electrons are tightly bound, behaving like insulators. Now, what happens as the temperature rises?

Some electrons get enough energy to jump to the conduction band, right?

Exactly! This concept is pivotal! Always remember that increased temperature gives electrons 'freedom' to conduct, much like 'Heating up the party!'
Energy Bands and Conductivity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s discuss energy bands. Who can define the conduction band in semiconductors?

The conduction band is the higher energy level where free electrons can move to conduct electricity?

Precise! Electrons jumping to the conduction band makes the semiconductor conductive. How does temperature influence this?

Higher temperatures give electrons more energy to escape to the conduction band. It’s like they’ve found the exit!

That's a fantastic analogy! Always think of it as 'Heating creates freedom' when we look at conductivity!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In the atomic structure of semiconductors, Silicon and Germanium each have four valence electrons which form covalent bonds in a crystalline lattice. At absolute zero, they behave like insulators, but as temperature rises, some electrons gain energy to transition from the valence band to the conduction band, illustrating the importance of atomic structure on electrical conductivity.
Detailed
Atomic Structure of Semiconductors
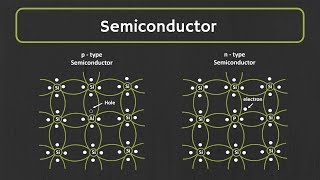
Semiconductors like Silicon (Si) and Germanium (Ge) are pivotal in the field of electronics due to their moderate electrical conductivity, which exists between that of conductors and insulators. At the atomic level, both Si and Ge possess four valence electrons that play a critical role in their ability to form covalent bonds within a crystalline lattice. As temperature decreases to absolute zero (0 K), these pure semiconductors behave much like insulators because all electrons are tightly bound within the valence band.
However, as the temperature begins to rise, thermal energy imparts enough energy to some electrons, enabling them to break free from their covalent bonds and jump into the conduction band. This transition is pivotal for the functionality of semiconductors, as it introduces free charge carriers that can conduct electricity. This section emphasizes the fundamental principles of atomic structure in semiconductors and sets the groundwork for understanding intrinsic and extrinsic semiconductors in subsequent sections.
Youtube Videos


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Valence Electrons in Semiconductors
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Silicon and Germanium have 4 valence electrons.
Detailed Explanation
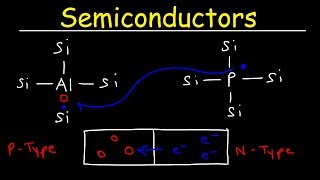
Silicon (Si) and Germanium (Ge) are important semiconductor materials. The term 'valence electrons' refers to the outermost electrons of an atom that can participate in forming chemical bonds. Each silicon and germanium atom has four valence electrons that play a crucial role in the electrical properties of these materials. These valence electrons are responsible for forming covalent bonds with neighboring atoms, which is fundamental to their function as semiconductors.
Examples & Analogies
Think of a semiconductor atom like a person at a party. Each person can form connections with friends (covalent bonds) based on how many hands they have available to shake (valence electrons). Since silicon and germanium have four hands (valence electrons), they are skilled at forming strong bonds with four other atoms in their structure.
Covalent Bonding and Crystalline Lattice
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● They form covalent bonds in a crystalline lattice.
Detailed Explanation
In the case of silicon and germanium, the atoms organize themselves in a crystalline lattice structure. This means that the atoms are arranged in a repeating pattern that extends in three dimensions. The covalent bonds formed between the valence electrons of adjacent atoms are very strong, contributing to the overall stability of the lattice. The ordered structure is essential for the semiconductors to exhibit their unique electronic properties.
Examples & Analogies
Imagine a well-organized seating arrangement at a wedding where each guest (atom) partners up with the person next to them (neighboring atoms) to create a beautiful and strong configuration (crystal lattice), where everyone is connected in a meaningful way (covalent bonds).
Behavior at Absolute Zero
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● At 0 K, pure semiconductors behave like insulators.
Detailed Explanation
At absolute zero (0 Kelvin), the energy of the atoms in a semiconductor is very low, and they are in their ground state. Under these conditions, there is not enough thermal energy available for the electrons to break free from their covalent bonds, thus acting as insulators. This means that they do not conduct electricity because there are no free charge carriers available to enable the flow of electric current.
Examples & Analogies
Consider a frozen lake in winter. When the temperature is very low, the surface (semiconductor) becomes hard and solid, preventing any boats (electric current) from moving across it. There are no ripples or waves (charge carriers) to allow the boats to float; everything is still.
Thermal Excitation and Conductivity
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● As temperature increases, some electrons gain enough energy to jump from the valence to the conduction band.
Detailed Explanation
When the temperature of a semiconductor rises, the atoms vibrate more energetically. This increased energy allows some of the valence electrons to gain enough energy to break free from their covalent bonds and move into the conduction band, where they can participate in conduction of electricity. This process creates 'holes' in the valence band, which can also conduct electricity since holes act as positive charge carriers.
Examples & Analogies
Think of heating a kettle of water. As the water gets hotter, molecules gain energy and start to move faster, eventually turning into steam (free electrons). The steam can flow freely (conduction), just as electrons can move when they gain sufficient energy to escape from their bonds.
Key Concepts
-
Valence Electrons: Electrons found in the outer shell of an atom responsible for covalent bonding.
-
Energy Bands: Distinct ranges of energy levels electrons can occupy within a solid.
-
Semiconductor Behavior at Temperature: Conductivity in semiconductors increases with temperature due to energy gain.
-
Covalent Bonding: Formation of strong connections between atoms through shared electrons.
Examples & Applications
In Silicon, four valence electrons form covalent bonds, resulting in a stable crystal lattice structure.
As temperature rises from absolute zero, electrons in Germanium gain energy and transition to the conduction band, facilitating conduction.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Valence at four, semiconductors score, heating brings energy, conductivity's core.
Stories
Imagine a party at absolute zero, where no one moves. But as the room heats up, everyone starts dancing freely, just like electrons moving into the conduction band.
Memory Tools
V.C.C - Valence, Covalent, Conductivity - to remember the main concepts of semiconductors.
Acronyms
S.O.C - Silicon, Oxygen, and Conductivity to recall the elements and their functions.
Flash Cards
Glossary
- Silicon (Si)
A widely used semiconductor material with four valence electrons.
- Germanium (Ge)
Another semiconductor material having four valence electrons, used in electronics.
- Covalent Bonds
Chemical bonds formed when atoms share pairs of electrons.
- Valence Band
The energy band that contains the valence electrons of a semiconductor.
- Conduction Band
The energy band where electrons can move freely to conduct electricity.
- Forbidden Energy Gap (Eg)
The energy range in a semiconductor where no electron states can exist.
- Intrinsic Semiconductor
A pure semiconductor without any dopants, showing baseline electrical properties.
Reference links
Supplementary resources to enhance your learning experience.
