Diffusion
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Diffusion in Environmental Context
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will discuss diffusion and its importance in environmental systems. Diffusion is the process by which substances move from areas of high concentration to areas of low concentration. Why do you think this is particularly relevant to environmental quality?

I think it’s important because pollutants can spread in water or air through diffusion.

Does this mean that the concentration decreases as substances diffuse?

Exactly! This movement balances concentrations, which can help us understand how contaminants disperse in sediments. Now, let's dive deeper!
Mass Balance and Diffusion
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's look at the mass balance in sediments. What do we think happens when we write a mass balance equation for a volumetric differential element?

I assume it shows the difference between what comes in and goes out?

Good! We denote the rate of accumulation, which is often not steady state. This means the input and output rates can differ, resulting in a net accumulation.

Does that mean we have to account for both fluid and solid phases?

Yes! That’s crucial, as actual diffusion occurs in fluid as well as solid phases. Let’s remember the acronym **D-FAS**, for Diffusion in Fluid and Solid.
Effective Diffusivity in Porous Media
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s consider effective diffusivity, especially in porous media. Can anyone explain how porosity might affect diffusion?

If a material is more porous, diffusion may be faster because pathways are clearer?

But what about when it's filled with solids? I think that slows it down, right?

Correct! As the effective diffusivity is affected by the medium's characteristics, we can use Millington-Quirk's expression to quantify this. Remember the mnemonic **PEACE** for Porosity Affects Effective COefficient.
Local Equilibrium Assumption
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let's explore the local equilibrium assumption. When can we say that the rates of adsorption and diffusion are balanced?

When the water flow velocity is low, and everything is diffusing at a similar rate?

Precisely! This allows us to simplify models significantly. However, this assumption cannot hold in more dynamic scenarios. Remember, it is crucial to judge the flow conditions.

So at higher velocities, we might have to model things differently?

Absolutely! Tying back with our earlier learning, it's all about understanding the dynamic between flow, adsorption, and diffusion. Let’s hold onto the acronym **DADS**, meaning Diffusion and Adsorption Dynamics Simplified!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section covers the concept of diffusion as it relates to environmental quality, focusing on the mass balance of sediments, the role of diffusion in the movement of substances into and out of sediment volumes, and the significance of effective diffusivity, especially in porous media. Fundamental equations and assumptions, such as the local equilibrium assumption, are also explored.
Detailed
Detailed Summary
This section provides an extensive overview of diffusion in environmental systems, focusing particularly on its relevance in sediment quality. It begins with a discussion on the mass balance equations in sediment volumes, noting crucial distinctions between steady-state and non-steady state conditions. The rate of accumulation is pivotal in this analysis since it is often non-zero, indicating the complex behaviors of diffusion dynamics.
A main highlight is the differentiation between diffusion within the fluid phase versus the solid phase. It elaborates on how diffusion rates depend on concentration gradients and the structured equations that define the movement of materials into and out of sediments. The teacher explains the terms used to express diffusion and how environmental factors, like porosity, affect the effective diffusivity of materials.
The concept of effective diffusivity becomes essential when discussing porous media. The section addresses equations such as the Millington-Quirk expression, which describes how effective diffusivity is influenced by porosity in various environmental contexts. Understanding these nuances is crucial for appreciating the complexities of contamination spread in environmental systems.
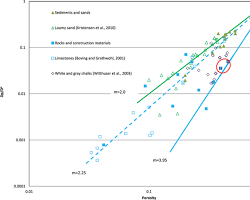
The session also delves into the local equilibrium assumption, which simplifies model complexities and assumes immediate mass transfer equilibrium between solid phases and pore fluids. However, it cautions that this assumption is applicable mainly under specific low-velocity transport conditions.
Lastly, the section prepares the groundwork for solving complex partial differential equations related to diffusion, connecting it to practical applications within environmental engineering and sediment studies.
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Mass Balance in Sediments
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So what we are doing? We do this normally in all box models kind of scenarios. We write again the mass balance in the sediment volume. This is delta x, delta y, delta z is the differential volume of the system. We write
∂C/∂t = (Rate_in - Rate_out) / V.
Now, here I can write other terms if I want to, yeah, I can write many things. Same as box model, I can write whatever is happening inside the system.
Detailed Explanation
This chunk introduces the concept of mass balance, which is crucial in understanding the dynamics of sediment systems. The mass balance equation essentially states that the change in concentration (∂C/∂t) within a given volume is equal to the difference between the rate of material entering and leaving that volume. The variables delta x, delta y, and delta z represent small changes in space dimensions, defining a tiny volume in which we analyze the mass flow. This framework is similar to what is used in simpler models, but it accounts for complex interactions in the sediment.
Examples & Analogies
Imagine a bathtub where some water is pouring in at the same rate as water is draining out. The mass balance equation here would help us figure out how the water level changes over time based on how much is coming in versus how much is going out. In sediments, it's like determining how pollutants accumulate based on how they enter and leave a soil volume.
Differential Accumulation and Diffusion
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The rate of accumulation is now, it is nonzero, it is not steady state, something is not going out in the same manner. So we have to write this in terms of diffusion. This is only by diffusion, so which means it is
∂C/∂t = D_A ∇^2C.
Detailed Explanation
In this chunk, the professor points out that unlike static systems, sediment systems experience a nonzero rate of accumulation. This means concentrations can change over time due to input from several sources, not just balance out from import and export. The diffusion term (D_A ∇^2C) describes how materials, such as pollutants, spread through the sediment, indicating that concentration changes are also driven by spreading out from areas of high concentration to areas of low concentration.
Examples & Analogies
Think of how a drop of food coloring disperses in water. At first, it’s concentrated in one spot, but over time, it spreads out until the color is uniform. Similarly, in sediments, if a pollutant is introduced, it will initially have a high concentration in one area, but over time will diffuse through the sediment, affecting larger areas.
Effective Diffusion in Porous Media
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This is called as effective diffusivity in a porous medium and it is a function of the porosity.
D_A = D_f * (Φ^n).
Detailed Explanation
Effective diffusivity refers to how well a substance can move through a porous medium (like soil) as opposed to through free fluid. It highlights that when dealing with porous materials, the presence of structures (like soil particles) can impede movement, making diffusion less efficient. The porosity (Φ) and an exponent (n) adjust the diffusivity to account for how much space is available for movement.
Examples & Analogies
Consider a sponge submerged in water. The water can easily move into the spaces between the sponge's fibers, but it takes longer for it to completely saturate the sponge. The sponge's structure limits how freely the water can spread, similar to how soil affects pollutant diffusion.
Local Equilibrium Assumption in Adsorption
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Local equilibrium assumption assumes that when a solid is near a fluid, the rate at which diffusion is occurring is almost the same order of magnitude at which this adsorption is happening.
Detailed Explanation
This assumption simplifies the complex interactions between solids (such as soil particles) and fluids (like water carrying pollutants) by saying that adsorption and diffusion happen simultaneously and at comparable rates. This means that as contaminants are diffusing into the solid matrix of sediment, they are also getting adsorbed (sticking) onto the solid particles at a similar pace.
Examples & Analogies
Imagine a sponge while you're pouring water over it. The water seeps in at nearly the same rate as it absorbs into the sponge's fibers. The local equilibrium assumption works under the premise that this balance remains constant, reflecting how pollutants interact with sediment.
Retardation Factor in Diffusion
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This term in the denominator is known as a retardation factor, which is equal to (ρ_s + K_d * ρ_w).
Detailed Explanation
The retardation factor explains how factors such as adsorption to solids (denoted by solid density ρ_s and distribution coefficient K_d) slow down the rate of diffusion. A large retardation factor indicates that the presence of solids substantially impedes the movement of contaminants through the water-soil interface.
Examples & Analogies
Think of a crowded hallway where people (representing pollutants) are trying to get from one end to the other. If the pathway is cluttered with obstacles (the soil particles acting as adsorbents), the people will move through the hallway much slower compared to a clear, unobstructed path. The retardation factor quantifies this slowdown caused by the solid structures.
Key Concepts
-
Mass Balance: The equation representing the difference between rates of inflow and outflow in a system.
-
Porosity: Critical for determining effective diffusivity within porous materials.
-
Local Equilibrium Assumption: A simplification that assumes instantaneous equilibrium between the fluid and solid phases.
Examples & Applications
In sediment environments, pollutants diffuse through water and sediment layers, influencing their distributions over time.
Effective diffusivity can be reduced in a contaminated aquifer due to solute adsorption onto soil particles, resulting in slower pollutant migration.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Diffusion flows from dense to sparse, moving fast in any environment’s course.
Stories
Imagine a crowded party where people move towards the exit as the music fades; this is similar to how molecules diffuse from crowded areas to open space.
Memory Tools
GERC - Gradient, Effective, Rate, Concentration; to remember the essential factors in diffusion.
Acronyms
PADS - Porosity Affects Diffusion Speed.
Flash Cards
Glossary
- Diffusion
The process by which molecules move from areas of high concentration to areas of low concentration.
- Effective Diffusivity
A measure that accounts for the obstacles faced by diffusing substances in a porous medium.
- Porosity
The measure of void spaces in a material, influencing how substances can diffuse through.
- Local Equilibrium
An assumption where the rates of mass transfer between solid and fluid phases are balanced.
- Mass Balance
The accounting of the mass entering, leaving, and accumulating in a defined system.
Reference links
Supplementary resources to enhance your learning experience.
