Rate of Accumulation
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Mass Balance in Sediments
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will explore the concept of mass balance in sediments. Can anyone tell me why mass balance is important in this context?

It helps us understand how substances move and accumulate in sediments.

Exactly! It allows us to track the inputs and outputs of materials in a sediment system. Now, what do we think the 'rate of accumulation' means in this context?

I think it refers to how quickly materials or contaminants build up in the sediments.

Correct! The rate of accumulation isn't constant; it can change based on environmental conditions. Let's break down the mass balance equation together.

Remember the acronym 'M.A.R.C.' for Mass Accumulation Rate Changes, which highlights key factors affecting accumulation rates.

Could you explain what factors can influence this rate?

Great question! Factors include sediment composition, fluid dynamics, and chemical interactions. We'll dive deeper into these shortly.

In summary, understanding mass balance and the rate of accumulation is essential for predicting contamination behavior in the environment.
Effective Diffusivity in Porous Media
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's talk about effective diffusivity. What do you think makes diffusion in porous media different from diffusion in other contexts?

Maybe it's because the material moves through tiny spaces in the sediment?

Exactly! The porous structure changes how easily substances diffuse. This leads us to define effective diffusivity, denoted as DA3. Why is this term so crucial?

It reflects how the porosity and solid interactions impact the diffusion process, right?

Yes! And do you remember the Millington-Quirk equation? It's a common expression for calculating effective diffusivity. What do we need to include in our models for accurate results?

Parameters like porosity, bulk density, and the partition coefficient!

Exactly! These parameters determine how effectively materials move in and out of the sediment. As a final note, can we think of an application of effective diffusivity in real-world scenarios?

Maybe in understanding pollution spread in water bodies?

That’s right! Effective diffusivity helps model how contaminants spread through sediments, allowing better environmental management.
Solid-Liquid Interactions in Rate of Accumulation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

So we understand the mass balance and effective diffusivity well. But how do solid-liquid interactions influence the rate of accumulation?

I guess it's about how materials adsorb on surfaces and how quickly they desorb?

Exactly! Those processes are critical. When we talk about adsorption and desorption, what do we mean in the context of our earlier discussions?

Adsorption is when a contaminant attaches to the sediment surface, while desorption is when it releases back into the fluid.

Well summarized! The balance between these processes determines the effective concentration in the sediments. Can anyone explain how conditions might change this balance?

Changes in temperature or fluid flow rates could affect the adsorption rates.

Indeed! Fluctuations in these factors can lead to variations in contaminant levels. Always keep in mind how dynamic these systems are!

To conclude, solid-liquid interactions are pivotal in understanding pollutant behavior in sediments.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section elaborates on the importance of understanding the rate of accumulation in sediment systems by introducing key concepts such as mass balance equations and diffusion processes. It emphasizes the interaction between solid and liquid phases and the necessity of considering effective diffusion coefficients in modeling environmental contaminants.
Detailed
Detailed Summary
This section delves into the rate of accumulation within sediment systems, emphasizing the mass balance in sediment volumes and its significance in environmental quality monitoring. The mass balance equation is adjusted for non-steady-state conditions, where the rate of accumulation is a crucial factor. The section identifies key distinctions in mass transfer processes, particularly the role of diffusion in transporting materials in and out of sediments. It introduces the term effective diffusivity, which accounts for the complexities of diffusion within porous media, crucial for understanding contamination spread in environmental engineering. The importance of parameters like porosity, bulk density, and adsorption is highlighted, along with various mathematical expressions for modeling these interactions. Rigorous definitions of terms and equations provide the foundational knowledge necessary to analyze sediment-water interfaces and the transport of contaminants effectively.
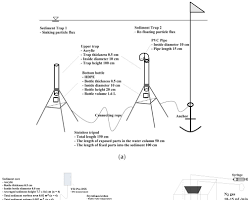
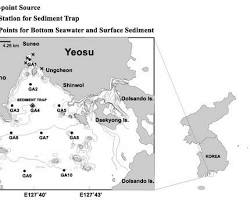
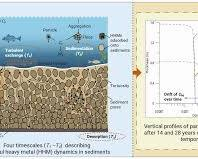
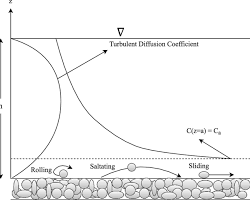
Youtube Videos










Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Rate of Accumulation
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So what we are doing? We do this normally in all box models kind of scenarios. We write again the mass balance in the sediment volume. This is delta x, delta y, delta z is the differential volume of the system.
Detailed Explanation
In this section, the professor introduces the concept of rate of accumulation within the context of system modeling, particularly using box models, which are simplified representations of a physical system. A mass balance is required to account for the inputs, outputs, and changes in mass within a defined volume, described by the differential dimensions (delta x, delta y, delta z).
Examples & Analogies
Think of a box (the system) in a muddy pond where you are measuring how much mud is entering and leaving that box over time. You need to keep track of how much mud is added, how much is taken out, and how much is settling inside the box to understand what happens to the mud over time.
Non-Steady State and Accumulation
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This is exactly what we did in box model except for two key differences. One is rate of accumulation is now, it is nonzero, it is not steady state, something is not going out in the same manner.
Detailed Explanation
The professor emphasizes that, unlike in steady-state conditions where inputs equal outputs, here we are dealing with non-steady conditions. This means that the quantity of substance in the sediment is changing over time, which necessitates a more dynamic consideration of mass transfer processes. The concept of 'rate of accumulation' is critical, as it signifies that whatever enters the system does not leave at the same rate.
Examples & Analogies
Imagine filling a bathtub (the box) with water while the drain is partially open. If you pour in water faster than it flows out, the water level in the tub rises, demonstrating a non-steady state where accumulation occurs. Conversely, if you manage to drain water at the same rate you're pouring it in, the bathtub remains steady.
Role of Diffusion in Accumulation
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
...what is it? So we have to write this in terms of the diffusion: the rate in and rate out is only by diffusion.
Detailed Explanation
In this part, the professor clarifies that both the input (rate in) and output (rate out) of substances within the model rely solely on diffusion processes. Diffusion refers to the movement of particles from areas of high concentration to areas of low concentration, and it plays a crucial role in determining how substances accumulate in the system.
Examples & Analogies
Consider a drop of food coloring in a glass of water. The way the color slowly spreads out into the clear water through diffusion is similar to how substances move in and out of a sediment system due to concentration gradients.
Total Mass Consideration
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So, therefore, we have to add this into a term called CT, in this case we have... which includes mass of A in the pore water + mass of A on the solids.
Detailed Explanation
Here, the professor introduces the term CT, which accounts for the total mass of substance A found both in the pore water and adhered to solid surfaces. This comprehensive view is essential for accurately representing the dynamics of accumulation, as it considers not just how much is dissolved in the water but also how much is absorbed onto the sediment particles.
Examples & Analogies
Think of CT as the total amount of sugar in your tea. If some sugar dissolves in the water and some sticks to the bottom of the cup, you need to account for both to know how much sugar you actually used.
Effective Diffusivity in Porous Media
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The diffusion is reduced because now for two reasons... diffusion of a material across a porous medium okay.
Detailed Explanation
In this segment, the professor elaborates on effective diffusivity—how diffusion behaves in porous materials. He explains that when diffusion occurs through a medium like soil, its pathways are not straightforward, which complicates the diffusion process due to factors like pore connectivity and the presence of solid particles. This understanding is vital for predicting how quickly substances can migrate through sediments.
Examples & Analogies
Imagine trying to walk through a dense forest (the porous medium) compared to on a wide-open field. In the forest, you encounter many obstacles (trees and underbrush) that slow your movement, just as solid particles slow down the diffusion of substances through a porous medium.
Key Concepts
-
Mass Balance: An essential equation reflecting inputs, outputs, and accumulation in environmental systems.
-
Effective Diffusivity: A critical parameter describing diffusion within porous media, influencing transport and reaction rates.
-
Adsorption and Desorption: Processes that represent the exchange of contaminants between solid surfaces and fluid phases, affecting accumulation rates.
Examples & Applications
In a sediment water interface, slow-moving contaminants may accumulate due to the adsorption process onto sediment particles, leading to varying concentrations in pore water.
Effective diffusivity can be modeled for a contaminated groundwater site to predict how pollutants might spread over time.
Porosity in soils influences how much water and nutrients can diffuse through, impacting plant growth and sediment quality.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In layers of land, pollutants can stand, Adsorption holds tight, while diffusion takes flight.
Stories
Imagine a sponge soaking water – this is like sediment adsorbing pollutants. As the sponge reaches full capacity, that’s like the rate of accumulation, limiting further absorption.
Memory Tools
Remember 'DADS' for Diffusion, Adsorption, Desorption, and Sedimentation; these processes interact in accumulation.
Acronyms
P.A.E.D. - Porosity, Adsorption, Effective diffusivity, Density - helps recall key parameters influencing sediment behavior.
Flash Cards
Glossary
- Rate of Accumulation
The rate at which materials or contaminants build up within a defined volume of sediment or system.
- Effective Diffusivity
A coefficient representing the rate of diffusion within a porous medium, accounting for the effects of the medium's structure on mass transfer.
- Mass Balance
An equation representing the relationship between the inputs, outputs, and changes in a system.
- Adsorption
The process by which atoms, ions, or molecules from a substance adhere to a surface.
- Desorption
The reverse process of adsorption, where a substance is released from a surface back into the fluid phase.
Reference links
Supplementary resources to enhance your learning experience.
