Droplets and Surface Tension
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Surface Tension
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're discussing surface tension. Can anyone tell me what surface tension is?

Isn’t it the force that makes the surface of a liquid resist an external force?

Exactly! Surface tension is a cohesive force at the surface of a liquid, allowing droplets to form. How do droplets maintain their shape?

They keep that shape because the surface tension pulls the molecules together.

Correct! This inward pull creates a balance, important in understanding how droplets behave under many conditions.

Are smaller droplets different from larger ones in how they behave?

Good question! Yes, the radius affects the pressure inside the droplet. Remember this with the acronym 'TIGHT' - Tension Increases Gradually with Height. Tensions can create variable droplet sizes.

So, if I understand right, the forces must balance out, right?

Absolutely. When we look at droplets, they're at equilibrium: the upward force from surface tension is equal to the downward pull of gravity.

Let's sum up: Surface tension allows droplets to hold shape, adjust size, and maintain forces in balance that lead to equilibrium.
Applications of Surface Tension
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s talk about the practical applications of surface tension. Can anyone mention an example?

Capillary action in plants!

Yes! Surface tension helps water move against gravity in plants. How does this work?

Water travels up tiny tubes due to the tension pulling it upwards.

That's right! And this is essential for nutrition. Remember: 'CAP' - Cohesion and Adhesion in Plants helps water rise.

How does that relate to our calculations in fluid dynamics?

Great connection! The equations we use for calculating height due to surface tension involve the radius of the tube as well as surface tension values.

Can flow measurement instruments use this concept?

Absolutely! Manometers utilize these principles to measure pressure differences in pipelines.

In summary, surface tension has applications in nature and technology, revealing its importance.
Deriving Key Formulas
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's derive the formula of capillary rise related to surface tension. Who remembers our balance of forces?

Isn’t it T equals mg?

Yes! Tension equals mass times gravity. Reference this with the 'T for Tension' in our formula! Can someone set up this equation for capillarity?

T = ρgAh?

Perfect! Now rearranging gives a deeper insight into how height relates to the diameters of tubes used. Remember 'DAWG,' Diameter Affects Water's Gain.

Is this applicable to both large and small diameters in droplets?

Yes! Both diameters are significant in calculating surface tension effects. Analyzing small and large droplets helps us to understand perspectives.

To recap, our derivation connects droplet behavior to calculations for height in different conditions, showcasing the interconnectedness of fluid mechanics.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
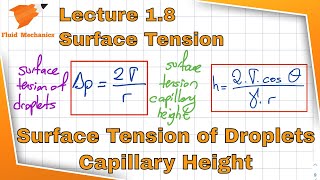
The section explores the balance of forces acting on droplets, particularly the upward and downward forces due to surface tension and weight. Equations illustrating the relationship between various fluid parameters are also highlighted, along with practical applications such as capillary rise in fluids.
Detailed
Detailed Summary
In this section, we delve into the concept of surface tension, particularly how it affects droplets and their behavior in liquids. The balance of forces acting on a droplet is analyzed, emphasizing the equilibrium condition where the upward force, predominantly from surface tension, counteracts the downward force related to the weight of the fluid.
- Equilibrium Conditions: We begin by establishing the equation for equilibrium, where the upward force due to surface tension (T) is equal to the downward gravitational force acting on the droplet.
- Mathematical Relationships: The derivation introduces essential formulas relating surface tension (σ), droplet diameter (D), and height (h) of capillarity. Two tension components from droplet interactions in different diameters are presented as a means to comprehend the mechanics of fluid columns in a manometer.
- Practical Applications: The discussion includes applications of these principles in real-world scenarios, such as in manometers for measuring pressure differences and analyzing phenomena like capillary action, which plays a crucial role in fluid mechanics.
- Observational Insights: Additionally, the narrative offers observations based on interactions with Japanese scholars, promoting systematic thinking and the importance of documentation in understanding scientific principles.
- Fluid Problems: The conclusion leads into practical examples and exercises involving fluid mechanics problems, which are crucial for consolidating the theoretical concepts covered in this section.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Equilibrium of Forces
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Now I have just equating this since is a equilibrium conditions in the so upward force is equal to the downward force.
Upward force = downward force
(T_1 + T_2) Cos(θ) * (D^2 - d^2)
The upward force is a surface tension force part, that what will act for a two different diameters.
Detailed Explanation
In this portion, we start by discussing the condition of equilibrium, where the upward force is balanced by the downward force. The upward force is derived from surface tension, which occurs at the interface of the liquid. When we talk about 'surface tension,' we refer to the cohesive forces at the surface of the liquid that cause it to behave as if it were covered by an elastic membrane. The formulas involve the angle (θ) and the diameters (D and d) that affect the tension's contribution to the force interacting with the system.
Examples & Analogies
Imagine a soap bubble, which forms due to surface tension. If we have two bubbles of different sizes, the force exerted by the air inside each bubble will depend on their radius. The bigger bubble exerts more upward force due to a larger curvature, and thus has more surface area interacting with the air.
Weight of the Fluid
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
We can compute the downward force which is the weight of the fluid. That what we confined by this the capillary rise.
Detailed Explanation
The downward force acting on the fluid is essentially its weight. This is important when understanding the behavior of liquids in narrow spaces, a phenomenon known as capillarity. The upward force, driven by surface tension, must balance this downward weight for a system to be in equilibrium. The height to which a liquid can rise in a narrow tube is directly related to this interplay of forces.
Examples & Analogies
Consider a straw in a glass of water. The water rises in the straw due to capillary action, which is the balance of the surface tension pulling the water upward against the weight of the water trying to fall back down.
Deriving Relationships
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If I just rearrange these terms, I will get this ones. That is what very basic way I will get it the relations between the capillarity height angle of contact and these two are the diameter of annular systems where you will have a and sigma stands for surface tensions.
Detailed Explanation
By rearranging the equations, we can derive important relationships between factors like capillary height, the angle of contact, and the diameters of the surfaces. Understanding these relationships helps us predict how a liquid will behave when confined in a small space, influencing applications from everyday tools like syringes to advanced technology in inkjet printers.
Examples & Analogies
When you see water climbing up a thin plant stem or a paper towel dipped in water, you are witnessing the effects of capillary action. The geometry of the stem or the paper towel affects how high the water climbs due to these relationships.
Key Concepts
-
Surface Tension: A force that allows droplets to maintain shape.
-
Equilibrium: The balance where surface tension equals weight.
-
Capillary Action: Movement of liquid in narrow spaces due to surface tension.
Examples & Applications
Example of a water droplet forming on a leaf due to surface tension.
Capillary action in plants allows water absorption from roots to leaves.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Droplets hold tight, thanks to surface tension's might.
Stories
Imagine a water droplet dancing on a leaf, held in place by its own strength, resisting the wind with surface tension.
Memory Tools
Remember 'CAP' - Cohesion and Adhesion allow Plants to rise, illustrating capillary action.
Acronyms
TIGHT
Tension Increases Gradually with Height in droplets.
Flash Cards
Glossary
- Surface Tension
The cohesive force at the surface of a liquid that allows it to resist external forces.
- Capillarity
The ability of a liquid to flow in narrow spaces against gravity.
- Equilibrium Condition
A state where the upward force due to surface tension balances the downward force from weight.
- Droplet
A small, spherical mass of liquid.
Reference links
Supplementary resources to enhance your learning experience.
