Conversion between temperature scales
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Temperature
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to talk about temperature. Can anyone tell me what temperature measures?

It measures how hot or cold something is!

That's a great start! Temperature actually measures the average kinetic energy of particles within a substance. More energy means higher temperature. Can anybody guess what the standard unit of temperature is?

Is it Celsius?

Celsius is one of them! The SI unit is actually Kelvin, but Celsius is most commonly used. Remember 'Kelvin K' if you're looking for the SI unit. Now, does anyone know the Fahrenheit scale?

I think it's used mostly in the USA!

Correct! Great observation!
Celsius to Fahrenheit Conversion
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's learn how to convert Celsius to Fahrenheit. One way to remember the formula is to think '9 over 5.' Can anyone tell me what the formula is?

Is it F = (9/5)C + 32?

Exactly right! This helps us convert temperatures when we need to, especially in the USA where Fahrenheit is used more. Who can give me an example?

If it’s 25 degrees Celsius?

Perfect! Let's calculate it together. What do we get?

F = (9/5) * 25 + 32, so F = 45 + 32, which equals 77!

Great job! 25 degrees Celsius equals 77 degrees Fahrenheit!
Celsius to Kelvin Conversion
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next up, we have the conversion from Celsius to Kelvin. Who remembers that formula?

K = C + 273!

That's right! Why do you think it's important to know Kelvin in scientific contexts?

Because Kelvin doesn't use negative values—like absolute zero!

Exactly! Let's do another example. What if we have 100 degrees Celsius?

So, K = 100 + 273—which equals 373 Kelvin!

Excellent! Now, remember these conversions well because they'll be useful in our next lessons!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section introduces the concept of temperature as the measure of average kinetic energy of particles and discusses the three primary temperature scales: Celsius, Fahrenheit, and Kelvin. It provides formulas for converting between these scales, emphasizing their applications in different contexts.
Detailed
Detailed Summary
In this section, we explore the concept of temperature, which quantifies the average kinetic energy of particles in a substance. The most widely used temperature scales—Celsius (°C), Fahrenheit (°F), and Kelvin (K)—are discussed, along with their specific applications. The section outlines the conversion formulas:
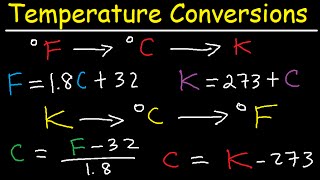
- Celsius to Fahrenheit: F = (9/5)C + 32
- Celsius to Kelvin: K = C + 273
These conversions are essential in various scientific and practical applications, illustrating the importance of understanding temperature scales in different fields, including science and daily life.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Celsius to Fahrenheit Conversion
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Celsius to Fahrenheit: F = \frac{9}{5}C + 32
Detailed Explanation
To convert a temperature in Celsius (C) to Fahrenheit (F), we use the formula: F = (9/5)C + 32. This formula means that for every degree Celsius, you multiply it by 9/5 and then add 32 to get the temperature in Fahrenheit. This conversion is useful because different regions use different temperature scales.
Examples & Analogies
Think of it like adjusting a recipe based on where you are cooking. If a recipe in a book from the US (which uses Fahrenheit) needs a certain temperature, you must convert it if you're using a Celsius temperature scale, much like adjusting measurements for teaspoons to tablespoons.
Celsius to Kelvin Conversion
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Celsius to Kelvin: K = C + 273
Detailed Explanation
Converting Celsius to Kelvin (K) is simpler. You just add 273 to the Celsius temperature. The Kelvin scale is mainly used in scientific contexts, especially in chemistry and physics, as it begins at absolute zero, the point where molecular motion stops.
Examples & Analogies
Imagine you are measuring the height of a ladder in two different ways. First, you measure in feet (Celsius), and then you convert it to meters (Kelvin) by simply adding a fixed amount to it (273), which allows you to understand it in a universally accepted scientific measure.
Key Concepts
-
Temperature: The measure of kinetic energy in particles.
-
Celsius to Fahrenheit Conversion: F = (9/5)C + 32.
-
Celsius to Kelvin Conversion: K = C + 273.
Examples & Applications
A temperature of 0°C corresponds to 32°F in Fahrenheit.
A temperature of 100°C is equivalent to 373 K in Kelvin.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Fahrenheit and Celsius are quite a pair, just remember 32 is where they share.
Stories
Imagine a scientist named Kelvin who discovered the absolute zero, where everything cools down even to hero!
Memory Tools
Fahrenheit starts at 32, while Celsius is no less true, just add up 273, to know the Kelvin too!
Acronyms
KCF for Kelvin, Celsius, and Fahrenheit—just remember to convert and relate!
Flash Cards
Glossary
- Celsius
A temperature scale where water freezes at 0°C and boils at 100°C.
- Fahrenheit
A temperature scale primarily used in the USA where water freezes at 32°F and boils at 212°F.
- Kelvin
The SI unit of temperature, starting from absolute zero. Water freezes at 273.15 K.
- Temperature
A measure of the average kinetic energy of the particles in a substance.
Reference links
Supplementary resources to enhance your learning experience.
