Laws of Thermodynamics
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Zeroth Law of Thermodynamics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, let's start with the Zeroth Law of Thermodynamics. This law defines a concept known as thermal equilibrium. Can anyone explain what that means?

Is it when two objects are at the same temperature?

Exactly! When two systems are in thermal equilibrium, they have no net heat exchange. So, if A is in equilibrium with B, and B with C, then A must also be in equilibrium with C. This helps us understand temperature measurement fundamentally. Remember, all measurements we take are based on this principle.

So, temperature is a way to express this equilibrium state?

Correct! Temperature serves as a scale to measure thermal equilibrium. Always remember: 'Temperature Ties'. Let's move on to the First Law.
First Law of Thermodynamics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

The First Law of Thermodynamics is all about energy conservation. Can anyone summarize it?

Energy can't be created or destroyed, right?

Yes! Energy is transformed from one form to another. The formula we use is ΔU = Q - W. Who can tell me what each symbol represents?

ΔU is the change in internal energy, Q is heat added, and W is work done by the system.

Great job! This law ensures that the energy within a system remains constant, just changing forms.

So, if I heat water, that's adding energy to the system?

Correct! You're adding heat (Q), increasing the internal energy (ΔU). Let’s summarize: 'Energy Exchanges, Never Disappears!' Now onto the Second Law.
Second Law of Thermodynamics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

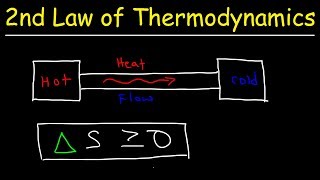
The Second Law deals with thermodynamic processes. It states that heat flows from a hotter body to a colder one. Can anyone tell me an example?

When you put a hot drink into a cold cup, it cools down?

Exactly! The heat moves from the drink to the cup. This law also introduces entropy, a measure of disorder. What happens to entropy in a closed system?

It increases because systems naturally favor disorder.

Correct! Remember: 'Heat Flows, Disorder Grows!' as a mnemonic for this principle. Let's conclude by discussing the Third Law.
Third Law of Thermodynamics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

The Third Law states that as temperature approaches absolute zero, the entropy of a perfect crystal approaches zero. What does this tell us about molecular motion?

At absolute zero, the molecules stop moving completely.

Exactly! This limit is important because it's a theoretical concept we can't actually reach. Just remember: 'Zero Motion at Zero Heat!' Let’s quickly revise all today’s key concepts!

Zeroth: Thermal equilibrium; First: Energy conservation; Second: Heat flow and entropy; Third: Entropy at absolute zero!

Fantastic recap, class!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section outlines the essential laws of thermodynamics: the Zeroth Law establishes thermal equilibrium, the First Law states that energy cannot be created or destroyed, the Second Law discusses the direction of heat transfer and entropy, and the Third Law refers to entropy as temperature approaches absolute zero.
Detailed
Laws of Thermodynamics
Thermodynamics is the branch of physics that deals with heat and temperature and their relation to energy and work. The laws of thermodynamics provide a framework for understanding how energy flows within physical systems. Here’s a breakdown of the four main laws:
- Zeroth Law of Thermodynamics: This law establishes the concept of thermal equilibrium. If system A is in thermal equilibrium with system B, and system B is in thermal equilibrium with system C, then system A is also in thermal equilibrium with system C. This is fundamental in establishing temperature as a measurable quantity.
- First Law of Thermodynamics (Law of Energy Conservation): It states that energy cannot be created or destroyed, only transformed from one form to another. Mathematically, it is expressed as ΔU = Q - W, where ΔU represents the change in internal energy, Q is the heat added to the system, and W is the work done by the system.
- Second Law of Thermodynamics: This law describes the natural direction of heat flow: it flows spontaneously from hotter objects to colder ones. This law also introduces the concept of entropy, which measures the disorder or randomness of a system, indicating that in any energy transfer, some usable energy will be dispersed, increasing the overall entropy of the universe.
- Third Law of Thermodynamics: This states that as the temperature approaches absolute zero (0 K), the entropy of a perfect crystal approaches a minimum value, which is taken as zero. However, absolute zero is practically unattainable, and refers to the limit of thermal motion.
Understanding these laws is crucial for many fields including physics, chemistry, and engineering, as they explain the behavior of energy in various processes.
Youtube Videos



Audio Book
Dive deep into the subject with an immersive audiobook experience.
Zeroth Law of Thermodynamics
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Zeroth Law of Thermodynamics: If two systems are each in thermal equilibrium with a third system, then they are in thermal equilibrium with each other.
Detailed Explanation
The Zeroth Law establishes a fundamental principle of thermodynamics, supporting the concept of temperature. It states that if system A is in thermal equilibrium with system C, and system B is also in thermal equilibrium with system C, then systems A and B are in thermal equilibrium with each other. This means they are at the same temperature and will not exchange heat when brought into contact.
Examples & Analogies
Think of it like three friends. If Alice is friends with Bob and Bob is friends with Charlie, then Alice and Charlie can be considered friends too, even if they haven't met. Similarly, if two systems are both 'friends' with a third system in terms of temperature, they are equally 'comfortable' with each other.
First Law of Thermodynamics
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● First Law of Thermodynamics (Law of Energy Conservation): Energy cannot be created or destroyed, only converted from one form to another.
○ ΔU=Q−W\Delta U = Q - W, where ΔU is the change in internal energy, Q is the heat supplied, and W is the work done by the system.
Detailed Explanation
The First Law of Thermodynamics emphasizes the conservation of energy, stating that energy in a closed system remains constant. Energy can change forms, such as from heat to mechanical work, but it cannot simply vanish. The formula ΔU = Q - W illustrates this law, where ΔU represents the change in the internal energy of the system, Q is the total heat added, and W is the work done by the system. If you add heat to a system (Q), part of that energy can go into doing work (W), while the remainder changes the internal energy (ΔU).
Examples & Analogies
Consider a car engine. The fuel you burn supplies heat energy (Q) that converts into mechanical work (W) to move the car forward. The remaining energy not used as work increases the internal energy of the engine, causing it to heat up. No energy is lost; it simply changes forms.
Second Law of Thermodynamics
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Second Law of Thermodynamics: Heat energy flows naturally from a body at a higher temperature to one at a lower temperature.
○ This law also introduces the concept of entropy (a measure of disorder).
Detailed Explanation
The Second Law of Thermodynamics describes the direction of heat transfer and introduces the concept of entropy, which gauges the disorder within a system. Essentially, heat will flow from a hot object to a cold object until both reach the same temperature, leading to increased disorganization or entropy in the universe. This law suggests that natural processes tend to evolve toward a state of greater disorder.
Examples & Analogies
Imagine mixing hot and cold water. When you pour hot water into cold water, it cools down, and the cold water heats up until they are both at the same temperature. This process reflects the second law as the energy spreads out to achieve balance, demonstrating the natural tendency from order (separate temperatures) to disorder (uniform temperature).
Third Law of Thermodynamics
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Third Law of Thermodynamics: As temperature approaches absolute zero, the entropy of a system approaches a minimum value.
Detailed Explanation
The Third Law of Thermodynamics posits that at absolute zero (0 Kelvin), a perfect crystal would theoretically have zero entropy, meaning it would be in a state of perfect order. In practical terms, this idea indicates that as we cool a system down toward absolute zero, its molecular motion slows significantly, decreasing its disorder (entropy) to near its lowest possible value. However, reaching absolute zero is physically unattainable.
Examples & Analogies
Think of a well-organized library. At room temperature, books might be slightly out of order, representing higher entropy. As you move to absolute zero, imagine all the books perfectly aligned and sorted, representing minimal entropy. However, in reality, you cannot fully achieve that perfect order since you cannot reach absolute zero.
Key Concepts
-
Zeroth Law of Thermodynamics: Establishes thermal equilibrium.
-
First Law of Thermodynamics: Energy cannot be created or destroyed.
-
Second Law of Thermodynamics: Heat flows from hot to cold, introducing the concept of entropy.
-
Third Law of Thermodynamics: Entropy approaches a minimum as temperature approaches absolute zero.
Examples & Applications
When two thermometers are in thermal equilibrium, they show the same temperature, illustrating the Zeroth Law.
If you heat a pot of water, the internal energy increases according to the First Law of Thermodynamics.
When ice melts, heat is absorbed without a temperature change, related to the Second Law.
As a sample cools down to absolute zero, the particles will come to a near standstill, demonstrating the Third Law.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When hot meets cold, energy flows; disorder grows as nature knows.
Stories
Once upon a time, there was a perfect crystal at absolute zero, where everything was still, a unique kingdom where disorder did not exist, but as the sun rose, energy flowed, and the dance of entropy began, and the kingdom became lively and chaotic.
Memory Tools
For remembering the laws: 'Zeroth - Equilibrium, First - Energy Stays, Second - Heat Flows, Third - Zero Entropy.'
Acronyms
ZEFZ
Zeroth
Energy (First)
Flow (Second)
Zero (Third)
to remember the sequence of laws.
Flash Cards
Glossary
- Zeroth Law of Thermodynamics
States that if two systems are each in thermal equilibrium with a third system, they are in thermal equilibrium with each other.
- First Law of Thermodynamics
States that energy cannot be created or destroyed, only transformed. Expressed as ΔU = Q - W.
- Second Law of Thermodynamics
States that heat energy flows from a body at a higher temperature to one at a lower temperature and introduces entropy.
- Third Law of Thermodynamics
States that as temperature approaches absolute zero, the entropy of a system approaches its minimum value.
- Entropy
A measure of disorder or randomness in a system, which tends to increase over time.
Reference links
Supplementary resources to enhance your learning experience.
