First Law of Thermodynamics
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to the First Law of Thermodynamics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome class! Today, we are diving into the First Law of Thermodynamics, which tells us that energy cannot be created or destroyed. It can only change forms. Can anyone give an example of energy transforming?

How about when we burn fuel in a car? The chemical energy in fuel is transformed into kinetic energy.

Exactly! That's a perfect example. So, can anyone tell me the formula that describes this transformation?

Is it ΔU = Q - W?

Yes! ΔU represents a change in internal energy, Q is the heat added, and W is the work done. This formula is crucial in thermodynamics! Remember it as 'Decrease from Work, Increase from Heat' to capture the relationships.
Internal Energy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's talk about internal energy. Can anyone tell me what it encompasses?

Does it include the energy of molecular movement?

Correct! Internal energy includes all forms of energy within a system. This means it considers energy associated with temperature and phase changes. What happens to internal energy when heat is added?

It increases, right?

Yes! And with increased internal energy, we can see consequences like increased temperature or even phase changes. This concept is crucial!
Heat and Work in Thermodynamics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s dive deeper. How does heat differ from work in thermodynamic terms?

Heat is the energy transferred due to temperature difference, and work is done when a force transfers energy.

Perfect! Heat flows from hot to cold, while work can be done in various forms, like lifting a weight. Who remembers a situation where both might occur together?

In a steam engine! It converts heat from burning fuel into work.

That's right! The transformation in a steam engine is a practical demonstration of the First Law in action.
Applications of the First Law
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

How does the First Law of Thermodynamics apply to heat engines?

It helps measure the efficiency of the engine.

Yes! Efficiency is literally how well an engine converts heat into work. The ratio of work done to heat supplied tells us a lot about its performance.

So, lower losses mean higher efficiency?

Correct! Remember, higher efficiency is always the goal for engineers and scientists.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section outlines the First Law of Thermodynamics, emphasizing the conservation of energy principle. It mathematically describes the relationship between internal energy, heat supplied to a system, and work done by the system, along with its implications in various thermodynamic processes.
Detailed
First Law of Thermodynamics
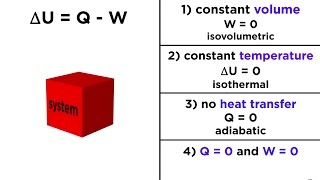
The First Law of Thermodynamics, also known as the Law of Energy Conservation, asserts that energy can neither be created nor destroyed; it can only change forms. This foundational principle is captured in the equation ΔU = Q - W, where ΔU represents the change in internal energy, Q denotes the heat added to the system, and W indicates the work done by the system.
Key Concepts Covered:
- Energy conservation in thermodynamic processes.
- Internal energy as an important state function.
- The mathematical representation of energy transformations.
The significance of this law lies in its application in understanding various physical and chemical processes, thus forming the groundwork for further studies in thermodynamics.
Youtube Videos


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to the First Law
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● First Law of Thermodynamics (Law of Energy Conservation): Energy cannot be created or destroyed, only converted from one form to another.
Detailed Explanation
The First Law of Thermodynamics is a fundamental principle in physics that states energy cannot be created or destroyed; it can only change forms. This means that the total energy in a closed system remains constant. For example, when chemical energy in fuel is converted to heat energy during combustion, the total amount of energy does not change; it simply transforms from one type to another.
Examples & Analogies
Think of energy like a budget of money. You can transfer money from one account (like savings) to another (like checking), but the total amount of money remains the same. Similarly, energy can shift between different forms, like moving from kinetic energy (energy of motion) to potential energy (energy of position), but the overall amount stays constant.
Understanding Internal Energy Change
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
○ ΔU=Q−W\Delta U = Q - W, where ΔU is the change in internal energy, Q is the heat supplied, and W is the work done by the system.
Detailed Explanation
The equation ΔU = Q - W describes how the internal energy of a system changes. ΔU (the change in internal energy) is equal to the heat added to the system (Q) minus the work done by the system (W). If you add heat to a system, its internal energy increases. Conversely, if the system does work (like pushing against a piston), it loses energy. Thus, the energy balance must account for both heat input and work output.
Examples & Analogies
Imagine a balloon. If you blow air into the balloon (adding heat), it expands and the internal energy increases. However, if you tie the end and release it, the balloon will do work on the air around it as it contracts, reducing its internal energy. The equation helps us keep track of whether the energy is increasing or decreasing.
Key Concepts
-
Energy Conservation: Energy can be neither created nor destroyed.
-
Internal Energy: Represents the total energy of a system.
-
Heat and Work: Heat is energy transfer, while work is energy moved through force.
-
Thermodynamic Processes: Understanding how energy transformations occur in real processes.
Examples & Applications
A car engine converts chemical energy from fuel into mechanical work and waste heat.
A refrigerator uses work to remove heat from inside, keeping the interior cool.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Energy flows, it's never lost, it just transforms, at any cost.
Stories
Imagine a magical tree that turns sunlight into food, which people then eat to gain energy and run, swim, or sprint—showing how energy transforms but never disappears.
Memory Tools
Use the acronym 'EQUIP': Energy (E), Quality (Q), Universal (U), Internal (I), Potential (P) to remember the key terms of the First Law.
Acronyms
Think of 'HEAT' to recall
Heat (H)
Energy (E)
Always (A)
Transforms (T).
Flash Cards
Glossary
- First Law of Thermodynamics
The law stating that energy cannot be created or destroyed, only transformed from one form to another.
- Internal Energy (U)
The total energy contained within a system, including kinetic and potential energies.
- Heat (Q)
The transfer of energy due to a temperature difference between systems.
- Work (W)
Energy transferred by a system due to an applied force.
- Efficiency
A measure of how much useful work is obtained from a certain amount of energy input.
Reference links
Supplementary resources to enhance your learning experience.
