Third Law of Thermodynamics
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to the Third Law
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're discussing the Third Law of Thermodynamics. Can anyone tell me what happens to a system as it approaches absolute zero?

Doesn’t it stop moving?

Good point! As temperatures drop, motion decreases. But more importantly, what else happens? What can you tell me about entropy?

Entropy measures disorder, right?

Exactly! The Third Law states that as we reach absolute zero, the entropy of a perfect crystal approaches zero. Who remembers why this matters?

So, it helps us understand how matter behaves at really low temperatures?

Yes! It also helps in calculating the absolute entropy of materials. Let’s summarize: entropy decreases as temperature approaches absolute zero.
Entropy and Absolute Zero
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Can anyone give me a definition of entropy in the context of thermodynamics?

I think it's about the amount of disorder in a system.

Exactly! More disorder means higher entropy. As we cool a substance toward absolute zero, it becomes more ordered, hence less entropy. Why is this significant in real-world applications?

Because it affects how we can use that energy in different technologies?

Yes! In fields like cryogenics, understanding these principles allows us to develop more efficient systems. Now, can anyone summarize what happens to a perfect crystal's entropy?

It approaches zero at absolute zero!

Correct! Let’s keep building on this knowledge.
Practical Implications of the Third Law
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

How do you think the Third Law of Thermodynamics influences our understanding of materials science?

It must help us figure out how materials behave at very low temperatures, like superconductors.

Exactly! Superconductors rely heavily on these principles. Can any of you think of other applications that might relate to low-temperature physics?

Cryogenic technologies, maybe?

Yes, and also in quantum computing, where low temperatures can help minimize energy loss or increase stability. Let's recap our last points!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
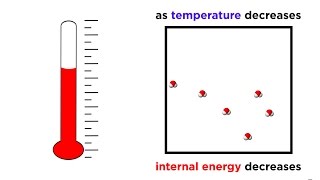
The Third Law of Thermodynamics highlights the behavior of systems as they approach absolute zero, emphasizing that the entropy (degree of disorder) of a perfect crystal approaches zero at this temperature. This law has significant implications for understanding thermodynamic processes and the behavior of materials at very low temperatures.
Detailed
Third Law of Thermodynamics
The Third Law of Thermodynamics posits that as the temperature of a system approaches absolute zero (0 Kelvin), the entropy, which quantifies the disorder or randomness of a system, approaches a minimum value. This implies that at absolute zero, a perfect crystal would have zero entropy, signifying a state of perfect order where all particles are in a fixed position. This law underlines the limitations of thermodynamic processes at extremely low temperatures and establishes a baseline for calculating the entropy of materials, thereby aiding in various applications such as cryogenics and quantum mechanics. In practical terms, the Third Law informs our understanding of energy distribution and efficiency in systems close to absolute zero, heavily impacting fields ranging from physical chemistry to solid-state physics.
Youtube Videos



Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to the Third Law of Thermodynamics
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The Third Law of Thermodynamics states that as temperature approaches absolute zero, the entropy of a system approaches a minimum value.
Detailed Explanation
The Third Law of Thermodynamics is a fundamental principle in physics. It tells us that when a system's temperature gets very close to absolute zero (which is 0 Kelvin or -273.15 degrees Celsius), the disorder or randomness of that system, known as entropy, decreases. In simpler terms, at absolute zero, a perfect crystalline structure would exist where every particle is in a specific, ordered state, resulting in very low entropy.
Examples & Analogies
Imagine a room filled with marbles of different colors all scattered around. At room temperature, they are all mixed up (high entropy). Now, as we cool the room down and push towards absolute zero, let's say we magically arrange them so that all red marbles are on one side and all blue marbles on the other (low entropy). This ordered state represents the idea of low entropy at very low temperatures!
Understanding Entropy and Absolute Zero
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Entropy is a measure of the disorder or randomness in a system, while absolute zero is the theoretical lowest temperature possible.
Detailed Explanation
Entropy can be thought of as a measure of how spread out or random the energy and matter in a system are. At higher temperatures, particles move more, and their positions are less predictable, resulting in higher entropy. As we cool down a substance towards absolute zero, the particles generally start to arrange themselves into more orderly structures, leading to lower entropy. However, reaching absolute zero itself is impossible according to the Third Law.
Examples & Analogies
Consider a box of kids' toys scattered all over the floor (high entropy). Now, imagine the kids start to clean up and put the toys back in their designated bins (lowering entropy). If we could theoretically freeze the room at absolute zero, all toys would perfectly be aligned, and the room would be utterly tidy — that’s the idea of perfect order!
Key Concepts
-
Third Law of Thermodynamics: As temperature approaches absolute zero, the entropy of a system approaches a minimum value.
-
Entropy: A measure of disorder in a system; at absolute zero, a perfect crystal's entropy approaches zero.
-
Absolute Zero: The theoretical lowest temperature achievable, marking the point where molecular motion ceases.
Examples & Applications
A perfect crystal at absolute zero has zero entropy, meaning all particles are in a fixed position with no disorder.
Superconductors exhibit unique properties at low temperatures that can be explained by the Third Law, emphasizing order in molecular structure.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
As temp drops low to zero's embrace, entropy finds a slower pace.
Stories
Imagine a room where toys are scattered everywhere—this is high entropy. Now, if all the toys are organized in one corner, it's like approaching absolute zero.
Memory Tools
A-B-C: Absolute - Banish - Chaos (As absolute zero is reached, chaos diminishes).
Acronyms
A-Z
Absolute Zero signifies an end to disorder; Totals Entropy = Zero.
Flash Cards
Glossary
- Entropy
A measure of disorder or randomness in a system; it quantifies how much thermal energy is unavailable for doing work.
- Absolute Zero
The lowest possible temperature, 0 Kelvin, where the motion of particles theoretically comes to a complete stop.
- Thermodynamics
The branch of physics that deals with the relationships between heat and other forms of energy.
Reference links
Supplementary resources to enhance your learning experience.
