Applications of Gas Laws
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Boyle's Law in Scuba Diving
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to discuss how Boyle's Law is applied in scuba diving. Can anyone tell me what Boyle's Law states?

I think it says that pressure and volume are inversely related when temperature is constant.

Exactly! So, when a diver goes deeper underwater, the pressure increases and their air volume decreases. This is crucial for managing air supply. Can you all remember it with the acronym 'PIV', Pressure Inversely Varies?

That helps! So if I go deeper, I need to be careful about how much air I use?

Correct! And if a diver ascends too quickly, they can experience decompression sickness because the volume of air expands rapidly. Always remember Boyle's Law while diving. Let's sum up: Boyle's Law helps divers manage air safely!
Charles's Law and Hot Air Balloons
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s move on to Charles's Law. What happens when the temperature of the gas in a hot air balloon increases?

The volume increases, and that makes the balloon rise!

Exactly! Remember, 'V = T' where Volume increases with Temperature when pressure is constant. Can anyone relate this to their experiences?

I saw a hot air balloon take off during a festival — it was amazing!

Great observation! This illustrates Charles's Law perfectly. To recap: hot air balloons rise because heated air expands, increasing the balloon's volume.
Gay-Lussac's Law in Tire Pressure
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's talk about how Gay-Lussac's Law applies to tire pressure. What does this law state?

It's about pressure and temperature being directly proportional when volume is constant.

Exactly! If it's a hot day, the pressure inside the tire increases. Anyone have an experience related to this?

I noticed my car tires seem to feel firmer after driving on a hot day!

That's right! So always monitor your tire pressure as heat can affect safety. Remember—'PT in the heat!' Keep that relationship in mind!
High Pressure in Cooking Gas Cylinders
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, we will discuss the concept of high pressure in cooking gas cylinders. Why do we store cooking gas under high pressure?

To keep more gas in a smaller volume!

Correct! It’s a practical application of gas laws, particularly Boyle's Law. Can anyone explain why it’s important to keep gas under high pressure?

So we can cook more efficiently without needing large tanks?

Exactly! And remember — higher pressure allows for better storage and efficiency; always handle with care as high-pressure gas is dangerous!
Human Respiration and Gas Laws
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let’s explore how gas laws relate to human respiration. Who can explain how these laws help us breathe?

Breathing involves changes in pressure and volume in the lungs.

Exactly! When we inhale, lung volume increases, decreasing pressure, and allowing air to flow in. How does this relate to the gas laws we've learned?

It's similar to Boyle's Law! More volume means lower pressure, right?

Correct! And just like gases, when we exhale, pressure increases, pushing air out. To summarize: gas laws are fundamental to understanding human respiration!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Gas laws have practical applications in various domains, including scuba diving tanks managing compressed air through Boyle's law, hot air balloons using Charles's law, and tire pressure management via Gay-Lussac's law. These laws also explain how cooking gas cylinders maintain high pressure for storage and the changes occurring during human respiration.
Detailed
Applications of Gas Laws
Gas laws are pivotal in understanding and managing various real-life scenarios. The primary applications discussed in this section illustrate how these laws function in everyday situations:
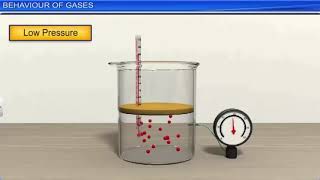
- Scuba Diving Tanks: Utilizes Boyle’s Law, which states that at constant temperature, the pressure of a gas is inversely proportional to its volume. This principle is crucial for divers to manage the air supply as they ascend and descend underwater.
- Hot Air Balloons: Rely on Charles’s Law, which posits that at constant pressure, the volume of a gas increases with its absolute temperature. This is evident in the heating of air that causes the balloon to rise.
- Tire Pressure: Changes are explained by Gay-Lussac’s Law, which states that at constant volume, the pressure of a gas is directly proportional to its temperature. Therefore, tire pressure increases on hot days.
- Cooking Gas Cylinders: High pressure is maintained to store large volumes of gas effectively, allowing for efficient cooking and heating.
- Human Respiration: Involves changes in gas volume and pressure, allowing for the inhalation and exhalation of air, which is also rationalized by the gas laws.
Overall, these applications showcase the relevance and importance of gas laws in practical scenarios.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Scuba Diving Tanks and Boyle's Law
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Scuba diving tanks: Use Boyle’s law to manage compressed air.
Detailed Explanation
When it comes to scuba diving, Boyle's Law helps us understand how the pressure of gas changes as it is compressed or allowed to expand. Boyle's Law states that at a constant temperature, the pressure of a given amount of gas is inversely related to its volume. This means that if you decrease the volume of the gas, the pressure increases. When divers descend underwater, the pressure surrounding them increases, causing the air in their tanks to compress, which is critical for safe breathing at different depths. Conversely, if they ascend, the pressure decreases and the volume of the gas expands, making it essential for divers to ascend slowly to avoid health risks associated with rapid pressure changes.
Examples & Analogies
Think about a balloon: if you squeeze it, it gets smaller (the volume decreases) but the air inside pushes out harder against the sides, increasing the pressure inside. Similarly, scuba tanks are built strong to withstand the high pressure of underwater environments.
Hot Air Balloons and Charles's Law
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Hot air balloons: Rise due to Charles’s law.
Detailed Explanation
Charles's Law states that the volume of a gas is directly proportional to its absolute temperature when the pressure is held constant. This means that as you heat the air inside a hot air balloon, the air expands, increasing its volume. Since the buoyant force acting on the balloon is greater than the weight of the balloon, it rises. The hotter the air inside the balloon, the larger its volume, and the more lift it generates, allowing people to enjoy flights high in the sky.
Examples & Analogies
Picture a campfire: when you heat a pot of water, the steam (which is a gas) created pushes up through the air. A hot air balloon works on the same principle, where the hot air inside expands and pushes upward, lifting the entire balloon.
Tire Pressure and Gay-Lussac’s Law
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Tire pressure: Increases with temperature (Gay-Lussac’s law).
Detailed Explanation
Gay-Lussac’s Law states that at constant volume, the pressure of a gas is directly proportional to its absolute temperature. This means if the temperature of the air in your tire increases, the pressure also increases, which is important for maintaining proper tire performance. For example, if you drive on a hot day, the heat generated from the road surface and from the tires themselves causes the air inside the tire to heat up, raising its pressure. It is crucial to check tire pressure regularly since over-inflated tires can lead to blowouts.
Examples & Analogies
Think about a soda can: if you leave it in a hot car, the gas inside becomes hot and builds up pressure, which could cause the can to burst if the pressure gets too high. This analogy demonstrates why it's important to monitor tire pressures in varying temperatures.
Cooking Gas Cylinders
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Cooking gas cylinders: High pressure maintained to store large volumes.
Detailed Explanation
In cooking gas cylinders, gases like propane or butane are stored under high pressure. The gas laws indicate that at higher pressures, the volume of the gas decreases. This allows a greater amount of gas to be stored in a smaller space, making it convenient for use in cooking and heating. When the gas is released from the cylinder, it expands rapidly and the pressure decreases, allowing it to flow out for cooking. Understanding this principle ensures that safe practices are maintained when handling these cylinders.
Examples & Analogies
Imagine packing a suitcase for a trip. The more you pack tightly and efficiently, the more items you can fit inside. Similarly, high-pressure cooking gas cylinders pack a large amount of gas into a compact space, so you can use it whenever needed.
Human Respiration and Gas Laws
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Human respiration: Involves volume and pressure changes explained by gas laws.
Detailed Explanation
Human respiration is an excellent example of how gas laws operate in biological systems. When we breathe in, our diaphragm moves down, increasing the volume of our lungs. According to Boyle's Law, as the volume increases, the pressure inside the lungs decreases, causing air to flow in. Conversely, when we exhale, the diaphragm moves up, reducing the volume and increasing the pressure, forcing the air out. This process is critical for delivering oxygen to our bodies and removing carbon dioxide effectively.
Examples & Analogies
Think of a syringe filled with air. When you pull the plunger back (increasing volume), air rushes in, but when you push it back in (decreasing volume), air exits out. Our lungs function similarly, drawing in air during inhalation and expelling it during exhalation.
Key Concepts
-
Boyle's Law: Pressure increases as volume decreases at constant temperature.
-
Charles's Law: Volume increases as temperature increases at constant pressure.
-
Gay-Lussac's Law: Pressure increases with temperature at constant volume.
-
Applications: Gas laws are applied in scuba diving, hot air balloons, tire maintenance, and cooking.
Examples & Applications
Scuba divers manage air pressure using Boyle's Law to ensure safe ascents.
Hot air balloons utilize Charles's Law when air is heated and expands.
Tire pressure increases in summer heat explained by Gay-Lussac’s Law.
Cooking gas is compressed to high pressure for effective storage in cylinders.
Human respiration involves changes in pressure and volume as we breathe.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When air is heated, it takes up space, by Charles's Law, it's a thrilling race!
Stories
A diver exploring the ocean felt the squeeze as he went deep, his tank’s air brewed with pressure so steep. But he knew Boyle's Law, and managed it well, now he ascends safely, returning to tell!
Memory Tools
PIV - Pressure Inversely Varies for Boyle's Law helps keep your diving safe!
Acronyms
GAS - Gay-Lussac’s law for tire pressure, Always monitor in the summer!
Flash Cards
Glossary
- Boyle's Law
At constant temperature, the pressure of a gas is inversely proportional to its volume.
- Charles's Law
At constant pressure, the volume of a gas is directly proportional to its absolute temperature.
- GayLussac's Law
At constant volume, the pressure of a gas is directly proportional to its absolute temperature.
- Ideal Gas
A gas that follows all gas laws under a range of conditions.
- Kinetic Theory of Gases
Theory that explains the behavior of gases based on particle motion.
Reference links
Supplementary resources to enhance your learning experience.
