Boyle’s Law
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Boyle's Law
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we'll talk about Boyle’s Law. It states that at a constant temperature, the pressure of a gas is inversely proportional to its volume. Can one of you explain what that means?

Does it mean if the volume decreases, the pressure increases?

Exactly! This relationship means that when you compress a gas, its pressure rises. Let’s think of a balloon. What happens when you squeeze it?

The air inside compresses, and it gets harder to squeeze, right?

Right! That's Boyle’s Law in action. A great way to remember this is the acronym PIV—Pressure increases, Volume decreases.

So it works as long as the temperature stays the same?

Exactly! That’s a key point. Let's summarize: Boyle's Law shows the inverse relationship between pressure and volume at constant temperature. Great contributions, everyone!
Mathematical Representation of Boyle's Law
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s look at the formula for Boyle’s Law: P × V = constant. How do you think we can use this formula in practical scenarios?

We can calculate what happens if we change the volume of a gas?

Correct! If you know the pressure and volume of a gas, you can find the other if you change one. What would the graph look like if we plotted this relationship?

It would be a curve, like a hyperbola?

Yes! It curves down, indicating that as one relationship increases, the other decreases. This is essential in understanding how gases behave under compression.
Real-World Applications of Boyle's Law
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s now explore how Boyle's Law applies in real life. Can anyone think of an example?

Like when you squeeze a syringe?

Perfect example! When you pull the plunger back, the volume increases, leading to a decrease in pressure, which draws liquid in. Any other examples?

What about deep-sea diving? The pressure changes as they go deeper, right?

Exactly! The air in a diver’s tank compresses, increasing pressure. That's why understanding Boyle’s Law is crucial for divers as it affects their breathing. Remembering PIV helps us apply this knowledge.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
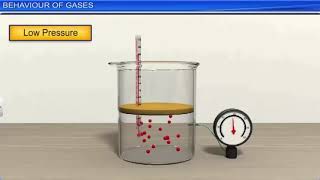
Boyle's Law describes the relationship between the pressure and volume of gas at constant temperature. According to this law, when the volume of a gas decreases, its pressure increases, and vice versa, mathematically represented as PV = constant. This fundamental principle illustrates how gases behave under compression.
Detailed
Boyle’s Law
Boyle's Law is a fundamental principle in gas physics that states: At constant temperature, the pressure of a given mass of gas is inversely proportional to its volume. This law can be mathematically expressed as the equation P × V = constant, indicating that if the volume (V) of a gas decreases, the pressure (P) increases proportionally, provided the temperature remains unchanged.
Graphical Representation: When plotted, the relationship between pressure and volume creates a hyperbola, indicating the inverse nature of their relationship. This concept is vital for understanding various real-world applications, such as the behavior of balloons or syringes, where squeezing reduces volume and increases pressure.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Statement of Boyle's Law
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
At constant temperature, the pressure of a given mass of gas is inversely proportional to its volume.
Detailed Explanation
Boyle's Law states that when the temperature of a gas remains constant, the pressure of that gas varies inversely with its volume. This means that if the volume of the gas decreases, the pressure increases and vice versa. The relationship is a key aspect of how gases behave under different conditions, and it can be mathematically represented. Essentially, you're compressing or expanding the gas, impacting how closely packed the molecules are, which directly affects the pressure they exert on their surroundings.
Examples & Analogies
Imagine a balloon filled with air. When you squeeze the balloon (decrease the volume), the air molecules inside are forced closer together. As they collide more frequently with the walls of the balloon, the pressure inside increases. Conversely, if you let go of the balloon, it expands, and the pressure decreases.
Formula Representation
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Formula: P × V = constant
Detailed Explanation
The formula P × V = constant encapsulates Boyle’s Law. 'P' represents the pressure of the gas, 'V' represents its volume, and the product of pressure and volume remains constant when temperature is unchanged. This means that if you know either pressure or volume, you can calculate the other using this relationship. For example, if the initial conditions of a gas are known, changes can be predicted accurately.
Examples & Analogies
Think of a syringe filled with air. When you pull back on the plunger (increase the volume), the pressure inside the syringe decreases. If you push the plunger in (decrease the volume), the pressure increases. You can manipulate one variable to affect the other, keeping the product constant.
Graphical Representation
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Graph: Pressure vs Volume → Curved (hyperbola)
Detailed Explanation
When plotting pressure against volume on a graph, the resulting curve forms a hyperbola. This visual representation illustrates the inverse relationship described by Boyle's Law. As volume increases, pressure decreases, leading to the characteristic curve. This helps us appreciate how one quantity directly affects the other, following the formula. Such a graphical approach can help students visualize relationship trends effectively.
Examples & Analogies
Picture a graph where the x-axis represents volume and the y-axis represents pressure. As you move right along the x-axis (increase volume), the graph descends sharply, showing that pressure drops. It’s like a seesaw—when one side goes up (volume increases), the other side goes down (pressure decreases).
Practical Example of Boyle's Law
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Example: When a balloon is squeezed, its volume decreases and pressure increases.
Detailed Explanation
This example illustrates Boyle’s Law at work. When a balloon is squeezed, you reduce the amount of space the air inside occupies, leading to an increase in pressure within the balloon as the gas molecules collide with the walls more forcefully. This is a straightforward application of the concept, making it easy to understand the direct effects of volume change on pressure in practical scenarios.
Examples & Analogies
Consider a simple practical situation: think about bicycle tires. When you pump air into the tires (decreasing volume inside the tire), the pressure increases. If the tire is punctured and begins to lose air, the volume inside the tire gradually increases, causing the internal pressure to drop. This visible effect underscores Boyle’s Law in action.
Key Concepts
-
Boyle's Law: The gas law describing the inverse relationship between the pressure and volume of a gas at constant temperature.
-
Inverse Proportionality: As volume decreases, pressure increases; represented mathematically as PV = constant.
Examples & Applications
An example of Boyle's Law can be seen when a balloon is squeezed: as the volume decreases, the pressure inside the balloon increases.
A practical implication is in syringes; pulling back the plunger increases volume and decreases pressure, allowing fluid to enter.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When the volume goes down, pressure goes up, just like a balloon that you squeeze with your cup!
Stories
Imagine a superhero who can control air pressure. When he squeezes the balloon, the air inside becomes angry and pushes back harder, that's how pressure increases with less space!
Memory Tools
PIV: Pressure Increases as Volume decreases.
Acronyms
PV = Pressure x Volume, a constant implies if one goes down, the other goes up!
Flash Cards
Glossary
- Boyle’s Law
A gas law stating that at constant temperature, the pressure of a given mass of gas is inversely proportional to its volume.
- Pressure
The force exerted by gas particles colliding with the walls of their container.
- Volume
The amount of space that the gas occupies.
Reference links
Supplementary resources to enhance your learning experience.
