Charles’s Law
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Charles's Law
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’re discussing Charles's Law. Who can tell me what this law states?

It says that the volume of a gas increases with temperature, right?

Exactly! At constant pressure, the volume of a gas is directly proportional to its absolute temperature in Kelvin. This means as the temperature goes up, the volume also goes up. Can anyone share the formula for this law?

Is it V/T = constant?

Yes! Great job! This formula helps us understand the relationship quantitatively. What does a graph of this law look like?

It’s a straight line, right? Like a linear graph?

Correct! A Volume vs. Temperature graph shows that direct proportionality as a straight line. Now, why do you think this is important?

It helps explain how hot air balloons work, right?

Exactly! When the air inside the balloon heats up, it expands, increasing the volume and allowing the balloon to rise. Let’s summarize: Charles’s Law shows that the volume of a gas increases with temperature at constant pressure – remember the formula V/T = constant!
Applications of Charles's Law
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s dive deeper! What are some real-life applications of Charles's Law?

The hot air balloon example is a big one!

Absolutely! And can anyone think of another example?

What about car tires? Their air expands when they heat up.

That’s right! As tires heat up from driving, the air inside expands, which can increase the pressure -- but we are focusing on volume. Does anyone have a memory aid to remember this law?

I remember the phrase 'Volume and Warmth Go Hand in Hand' helps me recall that volume increases with temperature!

Perfect mnemonic! So, repeating key concepts here: Volume increases with temperature at constant pressure, following the formula V/T = constant.
Graphical Representation and Understanding
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s analyze the graph of Charles’s Law. What shape do you see?

It looks like a straight line, starting from the origin.

That's correct! A straight line indicates a linear relationship. How does this relate to the formula we discussed?

It shows that if you plot volume on one axis and temperature on the other, they increase together.

Exactly! This visualization reinforces the concept. Can someone explain why the absolute temperature is important?

Because if we used Celsius, it wouldn’t show the same proportionality near zero!

Right! Always remember, volume must change with absolute temperature in Kelvin to maintain that relationship. Summarizing again: Charles’s Law shows us the direct relationship through a linear graph!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
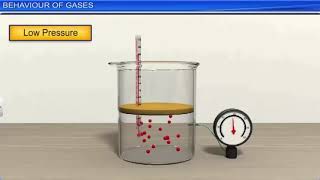
In Charles's Law, as the temperature of a gas increases, so does its volume, provided the pressure remains constant. This relationship can be represented mathematically and graphically, illustrating its significance in various applications, such as in hot air balloons.
Detailed
Charles’s Law
Charles’s Law is a fundamental principle in the study of gases, which states that at constant pressure, the volume of a gas is directly proportional to its absolute temperature measured in Kelvin. Mathematically, this relationship is expressed as:
Formula: V/T = constant
This means that if the temperature of a gas increases, the volume will also increase, as long as the pressure does not change. The graphical representation of Charles’s Law is a straight line on a Volume vs. Temperature graph, demonstrating a linear relationship. A practical example of this law can be observed with hot air balloons. When the air inside the balloon is heated, it expands, causing the volume to increase and resulting in the balloon rising. Understanding Charles’s Law is crucial for interpreting the behavior of gases under varying temperature conditions, tying back to the principles of the Kinetic Theory of Gases.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Statement of Charles’s Law
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
At constant pressure, the volume of a gas is directly proportional to its absolute temperature (in Kelvin).
Detailed Explanation
Charles’s Law tells us how the volume of a gas changes when the temperature changes, as long as the pressure stays the same. This means that if we heat the gas, its volume will increase, and if we cool it, the volume will decrease. The relationship is direct, meaning that higher temperatures lead to larger volumes.
Examples & Analogies
Think of a balloon: when you heat the air inside the balloon, the gas expands, making the balloon larger. However, if you put the balloon in a cold environment, the volume decreases as the air inside cools down.
Formula of Charles’s Law
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
V/T = constant.
Detailed Explanation
The formula V/T = constant shows that the ratio of volume (V) to temperature (T) is always the same for a specific amount of gas under constant pressure. This means that if you know the volume and temperature of a gas in one situation, you can predict what will happen in another situation as long as the pressure doesn't change.
Examples & Analogies
Imagine you’re a chef and you need to calculate how much dough to make. If you know how much dough you can put in the oven at a certain temperature, you can figure out how much to make if you change the oven temperature, keeping all other factors the same.
Graph of Charles’s Law
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Volume vs Temperature → Straight line.
Detailed Explanation
When we plot the relationship of volume on the y-axis and temperature on the x-axis, the result is a straight line that passes through the origin. This linear relationship reinforces the idea that as temperature increases, volume increases in a predictable manner.
Examples & Analogies
If you were to graph how your bicycle tire expands in the heat, you would see a straight line. More heat means greater volume of air, aligning perfectly with the concept of Charles’s Law.
Real-Life Example of Charles’s Law
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Hot air balloon rises because heated air expands, increasing volume.
Detailed Explanation
A hot air balloon is a practical example of Charles’s Law in action. When the air inside the balloon is heated, it expands and takes up more space. Since the volume of the heated air is greater compared to the cooler air outside the balloon, the balloon becomes buoyant and rises.
Examples & Analogies
Imagine you have a sealed bag filled with air. If you heat that bag on a stove, the air inside expands, causing the bag to puff up. Just like that bag, a hot air balloon rises as the heated air inside increases in volume compared to the cooler, denser air outside.
Key Concepts
-
Direct Proportionality: As temperature increases, volume also increases at constant pressure.
-
Absolute Temperature: Temperature must be measured in Kelvin for accurate proportionality.
-
Graph: The relationship between volume and temperature is represented as a straight line on a graph.
Examples & Applications
A hot air balloon rises as the air inside it is heated, expanding and increasing its volume.
In an old-fashioned thermometer, the increase in liquid volume corresponds to rising temperature.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When the warmth is high, the volume will fly, up to the sky, oh my!
Stories
Imagine a hot air balloon. When the sun heats the air inside, it expands and fills the balloon, causing it to rise high in the sky.
Memory Tools
V for Volume and T for Temperature, both go up together!
Acronyms
CVT
Charles's Volume Temperature relation.
Flash Cards
Glossary
- Charles's Law
The law stating that the volume of a gas is directly proportional to its absolute temperature at constant pressure.
- Absolute Temperature
The temperature measured on a scale where 0 is absolute zero, usually measured in Kelvin.
- Proportional
A relationship where one quantity varies directly with another.
- Volume
The amount of space occupied by a substance, commonly measured in liters or cubic meters.
Reference links
Supplementary resources to enhance your learning experience.
