Kinetic Theory of Gases – Postulates
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Composition of Gases
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’ll discuss the first postulate of the Kinetic Theory of Gases. Can anyone summarize what gases are composed of?

Gases are made up of molecules, right?

Correct! Gases consist of large numbers of small particles called molecules.

But how small are we talking about?

Excellent question! Molecules are incredibly small and exist in vast quantities. In fact, in a balloon, there could be millions of molecules! Remember, this vast number is what allows gas to fill any container.

Why can't we see them?

Molecular size is below the visibility limit for our eyes, but we can detect their effects like pressure and temperature changes.

So, to remember: **Gases = Large Numbers of Small Particles**!
Motion of Gas Particles
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Moving on to the next postulate, can anyone explain what the term 'continuous random motion' means for gas molecules?

Does that mean they are always moving around?

Exactly! Gas molecules are always in motion, and this motion is both continuous and random. They collide with each other and with the walls of their container.

Does that mean they're never at rest?

Yes, unless cooled to absolute zero, gas particles are never at complete rest! This motion is what allows gases to expand and fill their containers.

Think of it as a dance – molecules moving independently but constantly interacting! Remember: **Molecules = Always Dancing**.
Intermolecular Forces and Collisions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

The next postulate states that intermolecular forces are negligible except during collisions. What does that mean?

Does it mean that the forces between them don’t matter most of the time?

Precisely! The forces that attract or repel gas molecules are small compared to the energy of their motion. Hence, gases behave like independent particles unless they collide.

So, what happens during those collisions?

Good question! During these elastic collisions, energy and momentum are conserved. That’s why gas pressure is consistent!

To summarize: **Walls Don't Push Back, Until They Do!**
Pressure from Collisions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s explore how pressure is created. Does anyone know what causes gas pressure in a container?

It's from the molecules bumping into the walls, right?

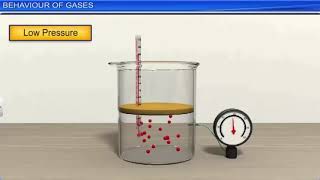
Absolutely! The pressure of a gas is the result of collisions between gas molecules and the walls of the container. More collisions mean higher pressure!

So if we increase the number of molecules, we get more pressure too?

"Exactly! That is why in a sealed syringe, the more we push down on the plunger, the fewer the available space, and the higher the pressure becomes.
Temperature and Kinetic Energy Relationship
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let's talk about how temperature relates to average kinetic energy. What do you think the connection is?

Does higher temperature mean faster molecules?

Exactly! Temperature is directly proportional to the average kinetic energy of gas molecules. When temperature rises, molecules move faster, increasing pressure.

So if we heat a gas in a sealed container, it will have higher pressure?

Yes, that’s right! This is a fundamental principle in thermodynamics. To help you remember: **Higher Temp = Higher Speed.**

Let’s summarize all key points: Gases are composed of particles in constant motion, forces are negligible, collisions are elastic, pressure is a result of those collisions, and temperature correlates with kinetic energy.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section outlines the five essential postulates of the Kinetic Theory of Gases: the composition of gases, the nature of particle motion, the insignificance of intermolecular forces, the elasticity of collisions, and the relationship between temperature and average kinetic energy. Together, these principles form the basis for understanding gas behavior.
Detailed
Kinetic Theory of Gases – Postulates
The Kinetic Theory of Gases is a fundamental concept in thermodynamics that explains the properties of gases through the following key postulates:
- Composition: Gases consist of a large number of small particles, known as molecules, which represent the basic unit of gas.
- Particle Motion: These gas molecules are in continuous and random motion, moving at various speeds and in different directions.
- Intermolecular Forces: The forces between these molecules are negligible except during collisions, meaning they do not significantly hinder the motion of the molecules under normal conditions.
- Elastic Collisions: Collisions between gas molecules are perfectly elastic, implying that there is no loss of kinetic energy during collisions. Thus, momentum and energy are conserved.
- Pressure and Temperature Relationship: The pressure exerted by a gas in a container is fundamentally due to the numerous collisions of the gas particles with the walls of the container. Additionally, the temperature of the gas is directly proportional to the average kinetic energy of its molecules, reinforcing the idea that as temperature increases, molecular motion becomes more vigorous and, consequently, increases the pressure.
These postulates form a coherent explanation of gas behavior and are foundational to the subsequent gas laws.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Composition of Gases
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Gases consist of large numbers of small particles (molecules).
Detailed Explanation
Gases are made up of an enormous number of tiny particles, known as molecules. These molecules are extremely small compared to the distances between them, which contributes to the unique properties of gases. The sheer quantity of these molecules means that they can fill any available space, which is why gases have no fixed shape or volume.
Examples & Analogies
Think of a gas like a large crowd of tiny people in a big hall. Even though there are many people (molecules), they are all spaced out and can move freely about the room (the container), much like gas molecules moving around in a space.
Motion of Gas Particles
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● These particles are in continuous, random motion.
Detailed Explanation
Gas molecules are constantly moving in all directions at different speeds. This continuous and random motion is fundamental to the behavior of gases. It explains why gases can fill up the entire volume of their container and why they are compressible and expandable.
Examples & Analogies
Imagine a busy train station with people moving all over randomly. Just like the people, gas molecules move freely and unpredictably in all directions, which is why they can quickly spread out to fill an empty space.
Intermolecular Forces
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Intermolecular forces are negligible except during collisions.
Detailed Explanation
In gases, the forces of attraction between individual molecules are very weak compared to the kinetic energy of the molecules themselves. This means that gas molecules do not stick together but rather move independently of one another, except at moments when they collide. During collisions, these forces momentarily come into play, but they do not significantly affect the overall motion of the gas.
Examples & Analogies
Think about a group of friends playing bumper cars at an amusement park. When they bump into each other (collisions), they may feel a slight jolt, but they don't stick together; they quickly move off in different directions, just like gas molecules.
Collisions of Gas Particles
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Collisions between molecules are perfectly elastic.
Detailed Explanation
When gas molecules collide, they do so in a perfectly elastic manner. This means that there is no loss of kinetic energy in the system during these collisions. Instead, the energy is transferred from one molecule to another, allowing the molecules to maintain their speed and direction after the collision.
Examples & Analogies
Imagine two billiard balls on a pool table. When they collide, they bounce off each other without losing any speed (assuming no friction). Similarly, gas molecules collide and bounce off each other without losing energy.
Pressure of Gases
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● The pressure of a gas is due to collisions with the walls of the container.
Detailed Explanation
Pressure in a gas is created by the force of gas molecules colliding with the walls of their container. Each time a molecule hits the wall, it exerts a small force. The collective effect of millions of these collisions results in a noticeable pressure on the surface of the walls. This is why gases exert pressure on their containers.
Examples & Analogies
Think of it like tiny rubber balls bouncing off the inside of a balloon. Each time a ball hits the balloon's wall, it pushes against it. The more balls (molecules) you have bouncing around, the greater the overall pressure inside the balloon.
Temperature and Kinetic Energy
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Temperature is directly proportional to the average kinetic energy of the molecules.
Detailed Explanation
Temperature is a measure of the average kinetic energy of the molecules in a gas. As the temperature increases, the average speed of the molecules also increases, meaning they have more kinetic energy. This relationship helps us understand how heating a gas can lead to increased pressure and faster movement.
Examples & Analogies
Consider a pot of water on a stove. As you heat the pot, the water molecules start to move faster and faster. The increased temperature means the molecules have higher energy, which you can observe when the water begins to boil and steam starts to rise.
Key Concepts
-
Composition of Gases: Gases are made up of a large number of small molecules.
-
Continuous Motion: Gas particles are always in random motion.
-
Negligible Intermolecular Forces: Forces between gas molecules are minimal except during collisions.
-
Perfectly Elastic Collisions: Collisions between molecules do not result in energy loss.
-
Pressure and Temperature Relationship: Pressure relates to collisions, temperature to kinetic energy.
Examples & Applications
When you inflate a balloon, you increase the number of gas molecules inside, which increases the pressure due to more collisions against the balloon's interior.
A hot air balloon rises because heating the air inside increases the average kinetic energy of the molecules, causing expands and reducing density.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Molecules are small, in motion they race,
Stories
Imagine a crowded dance floor where everyone is moving around randomly. The dancers bump into each other but never lose energy—they just keep dancing energetically, just like gas molecules in constant motion.
Memory Tools
Think of MICE (Molecules In Constant Energy) to remember that gas molecules are always in motion and their energy changes with temperature.
Acronyms
Use **PETC** (Pressure from Elastic collisions, Temperature's effect on Kinetic energy) to remember the key concepts that connect pressure, collisions, temperature, and kinetic energy.
Flash Cards
Glossary
- Gases
States of matter that have no fixed shape or volume, consisting of particles in motion.
- Molecules
Small particles that make up gases and other forms of matter.
- Kinetic Energy
The energy that a body possesses due to its motion, directly proportional to the temperature of the gas.
- Elastic Collisions
Collisions where there is no net loss of kinetic energy in the system.
- Pressure
The force exerted by gas molecules colliding with the walls of their container.
- Temperature
A measure of the average kinetic energy of the particles in a substance.
Reference links
Supplementary resources to enhance your learning experience.
