Behaviour of Perfect Gas and Kinetic Theory
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Gases
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will talk about gases. Can anyone tell me what makes gases different from solids and liquids?

Gases don't have a fixed shape or volume!

Exactly! Gases can expand and compress. What happens to the gas particles when we heat them?

They move faster!

Right again! This brings us to the Kinetic Theory of Gases, which we’ll dive into next.
Kinetic Theory of Gases – Postulates
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

The Kinetic Theory provides us with key postulates about gases. Who can share one of them?

Gases are made of a lot of tiny particles in constant motion.

Exactly! Can anyone tell me what happens during collisions between these particles?

They are perfectly elastic!

Correct! This means no kinetic energy is lost during collisions. Let’s remember this using the acronym 'CEMS' which stands for Continuous motion, Elastic collisions, Negligible forces, and Motion randomness.
Gas Laws
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s discuss some important gas laws. Can anyone share Boyle’s Law with us?

At constant temperature, pressure is inversely proportional to volume.

Well done! Can you give an example?

When you squeeze a balloon, it gets smaller and the pressure increases!

Great example! Now, what about Charles’s Law?

The volume of a gas is directly proportional to its absolute temperature.

Correct! Think about a hot air balloon rising: the heated air expands, increasing its volume. Let’s summarize the laws we’ve discussed.
Applications of Gas Laws
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we've covered the laws, let's look at applications. Can someone explain how Guy-Lussac's Law relates to tire pressure?

On hot days, the pressure inside the tire increases.

Correct! Can anyone think of another real-life application of these laws?

Scuba diving tanks! Boyle’s Law helps with managing compressed air while diving.

Excellent! Let’s wrap up with a brief summary of how these laws relate to our everyday experiences.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section delves into the characteristics of gases, the postulates of the Kinetic Theory, and key gas laws such as Boyle's, Charles's, and Gay-Lussac's. It emphasizes the relationship between temperature and kinetic energy, and provides practical applications of these concepts.
Detailed
Behaviour of Perfect Gas and Kinetic Theory
Overview
This section provides a comprehensive exploration of gases, focusing on their unique properties, the Kinetic Theory of Gases, and essential gas laws. The significance of understanding these concepts lies in their applications in real-world scenarios, from scuba diving to hot air balloons.
Key Points
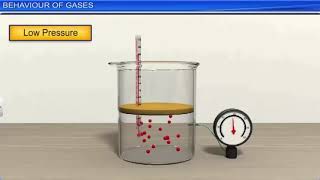
- Gases as a State of Matter: Gases differ from solids and liquids by having no fixed shape or volume, and they are easily compressible.
- Kinetic Theory of Gases: The behavior of gas molecules is essential to understanding gas laws. The postulates include:
- Gas particles are in constant, random motion.
- Intermolecular forces are negligible except during collisions.
- Collisions are perfectly elastic.
- Pressure results from collisions with container walls.
- Temperature relates directly to the average kinetic energy of particles.
- Gas Laws:
- Boyle’s Law states that at constant temperature, pressure and volume are inversely proportional.
- Charles’s Law states that at constant pressure, the volume is directly proportional to temperature in Kelvin.
- Gay-Lussac’s Law states that at constant volume, pressure is directly proportional to absolute temperature.
- Ideal Gas Equation: Combines all previous laws into the equation PV = nRT, which describes the state of an ideal gas.
- Applications: Real-world applications of gas laws include scuba diving, affecting tire pressure, and human respiration, which relies on pressure and volume changes.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Gases
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Gases are one of the fundamental states of matter.
● They have no fixed shape or volume and can be easily compressed or expanded.
● The behavior of gases is predictable through gas laws and the Kinetic Theory of Gases.
Detailed Explanation
Gases are a basic form of matter, similar to solids and liquids. Unlike solids, which have a fixed shape and volume, gases do not maintain a specific shape or volume. This means gases can fill any container they are placed in and can be squeezed into a smaller space (compressed) or allowed to spread out (expanded). Understanding how gases behave is crucial in science, particularly in chemistry and physics, which is why we have established gas laws and the Kinetic Theory of Gases that help us predict their behavior under different conditions.
Examples & Analogies
Think of a balloon filled with air. When you squeeze the balloon, the air inside compresses, and the balloon’s shape changes because the gas is being forced into a smaller volume. When you let go, the balloon expands back to its original shape as the gas expands to fill the space.
Kinetic Theory of Gases – Postulates
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Gases consist of large numbers of small particles (molecules).
● These particles are in continuous, random motion.
● Intermolecular forces are negligible except during collisions.
● Collisions between molecules are perfectly elastic.
● The pressure of a gas is due to collisions with the walls of the container.
● Temperature is directly proportional to the average kinetic energy of the molecules.
Detailed Explanation
The Kinetic Theory of Gases provides a framework to understand gas behavior at a molecular level. This theory states that gases are made up of numerous tiny particles (molecules) that move around freely in all directions. These particles are always in motion, and while they can interact with each other, the forces between them are very weak except when they collide. When they do collide, these collisions are perfectly elastic, meaning that no energy is lost—energy is transferred but the total energy remains constant. The pressure that we feel from a gas arises from these molecules hitting the walls of their container. Additionally, the temperature of a gas reflects how fast these molecules are moving, as higher temperatures mean that the average kinetic energy of the particles is greater.
Examples & Analogies
Imagine a room filled with bouncing balls. Each ball represents a gas molecule. As they bounce around the room, they collide with each other and with the walls, just like gas molecules do in a container. The faster the balls move (like the gas molecules at a higher temperature), the more pressure they exert on the walls of the room.
Applications of Gas Laws
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Scuba diving tanks: Use Boyle’s law to manage compressed air.
● Hot air balloons: Rise due to Charles’s law.
● Tire pressure: Increases with temperature (Gay-Lussac’s law).
● Cooking gas cylinders: High pressure maintained to store large volumes.
● Human respiration: Involves volume and pressure changes explained by gas laws.
Detailed Explanation
Gas laws are not just theoretical concepts; they have practical applications in our daily lives. For instance, scuba divers rely on Boyle's Law, which states that as they ascend and the pressure decreases, the volume of the air in their tank expands. Hot air balloons utilize Charles’s Law, where heating the air causes it to expand, making it less dense than the cooler air outside, resulting in the balloon rising. In car maintenance, understanding Gay-Lussac’s Law helps explain why the pressure in tires can increase on hot days. Similarly, cooking gas cylinders maintain high pressure, allowing for the storage of large amounts of gas in a small container. Even our breathing, where we inhaling and exhale air, can be explained using these gas laws, as the changes in pressure and volume in our lungs facilitate this process.
Examples & Analogies
Consider a scuba diver coming to the surface. As he rises, the water pressure around him decreases, allowing the air in his tank to expand. Without applying Boyle’s Law, he might not realize that he needs to control his ascent to prevent the tank from bursting due to the increased volume of air as pressure decreases.
Key Concepts
-
Pressure: The force exerted by gas particles colliding with a surface.
-
Volume: The amount of space occupied by a gas.
-
Temperature: A measure of the average kinetic energy of gas particles.
-
Elastic Collisions: Collisions where no kinetic energy is lost.
Examples & Applications
When a hot air balloon is heated, the air inside expands causing the balloon to rise (relates to Charles's Law).
A scuba diver's tank is compressed, demonstrating Boyle's Law when the volume of air decreases under pressure.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Gases on the fly, shape and volume go high; Squeeze them tight, pressure's height!
Stories
Imagine a balloon at a party. When you heat the room, the balloon expands and floats high—just like Charles's Law! But if you squeeze it, watch how the pressure spikes, following Boyle’s insight.
Memory Tools
P-V Inverse for Boyle, V-T direct for Charles, P-T direct for Gay-Lussac.
Acronyms
In Kinetic Theory, remember 'CCEM' for Continuous motion, Collisions, Elasticity, and Motion randomness.
Flash Cards
Glossary
- Perfect Gas
An ideal gas that follows all gas laws at all conditions of temperature and pressure.
- Kinetic Theory
A theory that explains gas behavior in terms of particles in motion.
- Boyle's Law
At constant temperature, pressure and volume of a gas have an inverse relationship.
- Charles's Law
At constant pressure, the volume of a gas is directly proportional to its absolute temperature.
- GayLussac's Law
At constant volume, pressure is directly proportional to temperature.
- Ideal Gas Equation
The formula PV = nRT, which relates the pressure, volume, and temperature of an ideal gas.
Reference links
Supplementary resources to enhance your learning experience.
