Introduction to Gases
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Properties of Gases
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're starting with gases. What do you think makes gases different from solids and liquids?

Gases don’t have a fixed shape or volume.

Great! Yes, gases are unique because they will expand to fill any space. Can anyone tell me why gases can be compressed easily?

Because they have a lot of empty space between the particles.

Exactly! That space allows them to be packed closer, which is why we can compress them. Remember the phrase 'expand and compress' for gases!
Gas Laws Overview
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s talk about gas laws! Who can name one gas law we've learned about?

Boyle's Law!

Right! Boyle's Law tells us how pressure and volume are related. When one goes up, the other goes down at constant temperature. Can anyone provide a real-life example?

Like when you squeeze a balloon; the volume decreases, and the pressure increases.

Exactly! Remember the acronym P-V for Pressure-Volume. The behavior of gases can often be predicted with these laws.
Application of Gas Laws
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's connect our learning to real-life situations. Can anyone mention an application of these gas laws?

Hot air balloons use Charles’s Law.

Well done! Warm air expands, which makes the balloon rise. If you think of hot air as 'going up' with temperature, keep in mind 'Charley climbs on hot air!' as a mnemonic.

What about scuba diving tanks?

Perfect! Boyle's Law helps divers understand how the pressure changes with depth. Remember, 'As you dive deep, pressure creeps!'
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
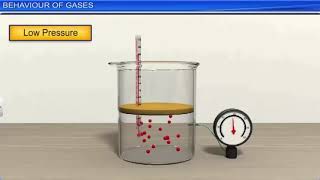
This section introduces the properties of gases, explaining their lack of fixed shape or volume and their ability to be compressed or expanded. The predictable nature of gas behavior is highlighted through various gas laws and the Kinetic Theory of Gases.
Detailed
Introduction to Gases
Gases represent a fundamental state of matter characterized by their ability to fill any container's shape and volume, reflecting their lack of a fixed form. Unlike solids and liquids, gases can be compressed and expanded significantly, which allows them to adapt to the volume of their containers. Their behavior is governed by the gas laws, each describing the relationship between pressure, volume, and temperature. Additionally, the Kinetic Theory of Gases provides a molecular perspective, suggesting that gases consist of a vast number of small, moving particles that interact through negligible forces except during collisions. Understanding these principles is crucial for analyzing real-world applications such as medical gas use, weather patterns, and industrial processes.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Fundamental States of Matter
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Gases are one of the fundamental states of matter.
Detailed Explanation
This point emphasizes that gases are one of the three primary states of matter, alongside solids and liquids. Gases differ in that they do not have a defined shape or volume; they take the shape and volume of their container. Understanding that gases are fundamental helps to grasp their unique properties and behaviors in contrast to the other states of matter.
Examples & Analogies
Think of how water behaves as liquid in a cup - it conforms to the shape of the cup. Similarly, if you take an air-filled balloon, the air inside doesn't have a specific shape or volume of its own but adapts to the balloon's shape.
Properties of Gases
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● They have no fixed shape or volume and can be easily compressed or expanded.
Detailed Explanation
Gases possess unique properties that allow them to expand to fill any available space and compress into a smaller volume. Unlike solids, which maintain a specific shape and volume, or liquids, which have a definite volume but not a fixed shape, gases are highly flexible. This characteristic is due to the large amount of space between gas particles, which enables them to move freely and quickly.
Examples & Analogies
Imagine filling a balloon with air. The balloon expands as you blow air into it, demonstrating that air (a gas) takes on the shape and volume of the balloon. When you squeeze the balloon, you compress the gas inside, showing its ability to change volume easily.
Predicting Gas Behavior
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● The behavior of gases is predictable through gas laws and the Kinetic Theory of Gases.
Detailed Explanation
The behavior of gases can be understood and predicted using specific mathematical relationships called gas laws, such as Boyle's Law, Charles's Law, and Gay-Lussac’s Law. The Kinetic Theory of Gases complements these laws by providing a microscopic view, explaining how particles in a gas move and interact. This predictability is crucial for various applications in science and engineering.
Examples & Analogies
Consider how you can predict what happens when you heat a gas in a sealed container. As the temperature increases, the pressure inside the container rises because, according to the gas laws, heating causes gas particles to move faster and collide more often with the container walls. This predictable behavior is similar to how you can anticipate the contents of a boiling pot of water when you increase the heat.
Key Concepts
-
Gases do not have a fixed shape or volume.
-
Gases can be compressed and expanded.
-
Gas laws help predict the behavior of gases, connecting pressure, volume, and temperature.
-
The Kinetic Theory explains how gas particles behave.
Examples & Applications
A balloon filled with air expands when heated, demonstrating Charles’s Law.
A sealed soda can experiences increased pressure when shaken, illustrating Boyle's Law.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Gases can expand and contract, always on the move, that's a fact!
Stories
Imagine a balloon that gets warm in the sun. It starts to grow, expanding just for fun!
Acronyms
GEM
Gases Expand and Move!
Flash Cards
Glossary
- Gas
A state of matter that has no fixed shape or volume.
- Gas Laws
Equations that describe the relationships between pressure, volume, and temperature in gases.
- Kinetic Theory of Gases
A theory that explains the behavior of gases in terms of particles in motion.
Reference links
Supplementary resources to enhance your learning experience.
