Kinetic Energy and Temperature
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Kinetic Energy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will discuss kinetic energy and its relation to temperature. Can anyone tell me what kinetic energy means?

Isn't it the energy of motion?

Exactly! Kinetic energy is the energy that an object possesses due to its motion. In gases, this means that molecules are constantly moving.

So, do all gases have the same kinetic energy?

Good question! No, the average kinetic energy varies with temperature. As temperature increases, what happens to the molecules?

They move faster!

Correct! So remember, increased temperature means increased kinetic energy. A good way to remember this is 'hotter, faster'.
Temperature's Impact on Kinetic Energy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let’s talk about how temperature influences kinetic energy. Who can explain how this occurs?

If the temperature rises, the molecules get more energy and speed up?

Exactly! That’s a perfect explanation. This relationship can be summarized as KE_avg ∝ T. Can anyone elaborate on why this matters?

It helps us understand why heating a gas can increase its pressure!

Precisely! Pressure increases as molecules collide more frequently with the walls of their container. Remember the acronym 'K.E.T.'—Kinetic Energy and Temperature!
Practical Implications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Can anyone think of a practical example that demonstrates the relationship between kinetic energy and temperature?

Like how the pressure in a car tire changes on a hot day?

Absolutely! As the temperature rises, the pressures in the tire increase due to faster moving air molecules. That shows us the principle we just discussed.

So, heating gases increases their pressure, which we use in things like balloons and scuba tanks?

Exactly! Great observation. This understanding is crucial for various applications in science and daily life.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
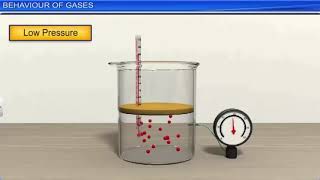
In this section, the link between temperature and the average kinetic energy of gas molecules is explored. It highlights that as the temperature of a gas rises, its molecules move faster, leading to increased pressure in a closed container, illustrating fundamental principles of kinetic theory.
Detailed
Kinetic Energy and Temperature
In this section, we delve into the relationship between the kinetic energy of gas molecules and temperature. According to the Kinetic Theory of Gases, the average kinetic energy
of gas molecules is directly proportional to the temperature (
KE_avg ∝ T). This means that as the temperature of a gas increases, the average kinetic energy of its molecules rises, causing them to move more rapidly.
This elevated molecular motion results in an increase in pressure within a closed container, explaining why heating a gas in such conditions leads to higher pressure levels. This section underscores the importance of understanding kinetic energy in relation to temperature, as it provides insight into gas behavior under varying thermal conditions.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Average Kinetic Energy and Temperature Relationship
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● The average kinetic energy of gas molecules increases with temperature.
● Relation: KE_avg ∝ T
Detailed Explanation
This chunk explains how the average kinetic energy (KE_avg) of gas molecules is directly related to temperature (T). As the temperature of a gas increases, the speed at which its molecules move also increases. This is because temperature is a measure of how much energy the molecules possess; higher energy means faster movement. Therefore, we can say that if we were to plot average kinetic energy against temperature, the two would show a proportional relationship.
Examples & Analogies
Think of gas molecules like people at a party. When the music is slow (low temperature), people move slowly and are less active. But when the music gets faster (higher temperature), people start dancing vigorously and moving around quickly. Similarly, as the temperature rises, gas molecules move faster.
Impact of Temperature on Molecular Movement
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● As temperature rises, molecules move faster, increasing pressure and energy.
Detailed Explanation
When we heat a gas, not only does the average kinetic energy of the molecules increase, but their individual speeds also increase. Faster-moving molecules collide with the walls of their container more forcefully and more frequently. This increased movement leads to higher pressure in a sealed container, as defined by the kinetic theory of gases. Essentially, if you think of the molecules as little balls bouncing around, more energy means they bounce off the walls with greater force, leading to an increase in pressure.
Examples & Analogies
Imagine a group of kids blowing up a balloon. Initially, when they blow gently (lower temperature), the balloon expands slowly. But if they blow harder (higher temperature), the air inside the balloon moves more vigorously, causing it to expand faster and potentially increasing the pressure inside it. This reflects how heating gas can lead to higher pressure.
Heating a Gas and Pressure in a Closed Container
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
● Demonstrates why heating a gas increases pressure in a closed container.
Detailed Explanation
In a closed container, heating the gas directly affects its molecules. As the gas is heated, the molecules gain energy, leading to faster movement. This increase in speed means they collide with each other and the walls of the container more often and with greater force, which raises the overall pressure within the container. This relationship explains why we're cautious about heating gases in sealed environments, such as pressure cookers, as the pressure can build up to dangerous levels.
Examples & Analogies
Think about a soda can. When you shake it, the gas inside starts moving faster due to the extra energy, and if you were to open it immediately, the pressure from the gas rushing out can create a mess. The heating of the gas inside can lead to similar pressure increases, showing how energy influences the behavior of gases.
Key Concepts
-
Kinetic Energy: The energy associated with the motion of particles in a gas.
-
Average Kinetic Energy: Dependent on temperature, as temperature increases, so does average kinetic energy.
-
Temperature and Pressure: Understanding the relationship between temperature and the pressure exerted by gas molecules.
Examples & Applications
A sealed gas container will experience increased pressure when heated, due to faster-moving gas molecules.
Hot air rises because the warm air expands and has higher kinetic energy, allowing it to move upward.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Hotter means faster, kinetic energy grows, pressure increases, everyone knows!
Stories
Once there was a gas in a closed jar. It got hot and started to race, bumping hard into the walls, increasing the pressure in its place!
Memory Tools
Remember K.E.T. – Kinetic Energy and Temperature! As T increases, K.E. increases too!
Acronyms
K.E.T - Kinetic Energy (K.E.) increases with Temperature (T).
Flash Cards
Glossary
- Kinetic Energy
The energy that an object possesses due to its motion.
- Average Kinetic Energy
The mean energy of motion of particles in a gas, directly related to temperature.
- Temperature
A measure of the average kinetic energy of the particles in a substance.
- Pressure
The force exerted by gas molecules when they collide with the walls of their container.
Reference links
Supplementary resources to enhance your learning experience.
